Chapter 20: Alkanes and Alkyl Halides
Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry
by Gregory Anderson; Jen Booth; Caryn Fahey; Adrienne Richards; Samantha Sullivan Sauer; and David Wegman
Chapter 20 Contents
- 20.1 Characteristics of Alkanes
- 20.2 Alkane Formulas
- 20.3 Isomers of Alkanes and IUPAC Nomenclature
- 20.4 Cycloalkanes
- 20.5 Halogenated Alkanes
- 20.6 Reactions of Alkanes
- Chapter 20 – Summary
- Chapter 20 – Review
- Chapter 20 – Infographics
Except where otherwise noted, this OER is licensed under CC BY-NC-SA 4.0
Please visit the web version of Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry to access the complete book, interactive activities and ancillary resources.
In this chapter, you will learn about:
- Characteristics of alkanes
- Alkane formulas
- Isomers of alkanes and nomenclature
- Cycloalkanes
- Alkyl halides
- Reactions of alkanes
To better support your learning, you should be familiar with the following concepts before starting this chapter:
- Covalent Bonding (from Chapter 11: Chemical Bonding)
- Molecular structure and VSEPR (from Chapter 11: Chemical Bonding)
- General concepts of organic chemistry (from Chapter 19: Organic Chemistry)
As you just learned, there is a wide variety of organic compounds containing different functional groups. However, all organic compounds are hydrocarbons, they contain hydrogen and carbon. The general rule for hydrocarbons is that any carbon must be bonded to at least one other carbon atom, except in the case of methane which only contains one carbon. The bonded carbons form the backbone of the molecule to which the hydrogen atoms (or other functional groups) are attached. Refer to Appendix A: Key Element Information for more details about carbon.
Hydrocarbons with only carbon-to-carbon single bonds (C–C) are called alkanes (or saturated hydrocarbons). Saturated, in this case, means that each carbon atom is bonded to four other atoms (hydrogen or carbon)—the most possible; there are no double or triple bonds in these molecules.
Saturated fats and oils are organic molecules that do not have carbon-to-carbon double bonds (C=C).
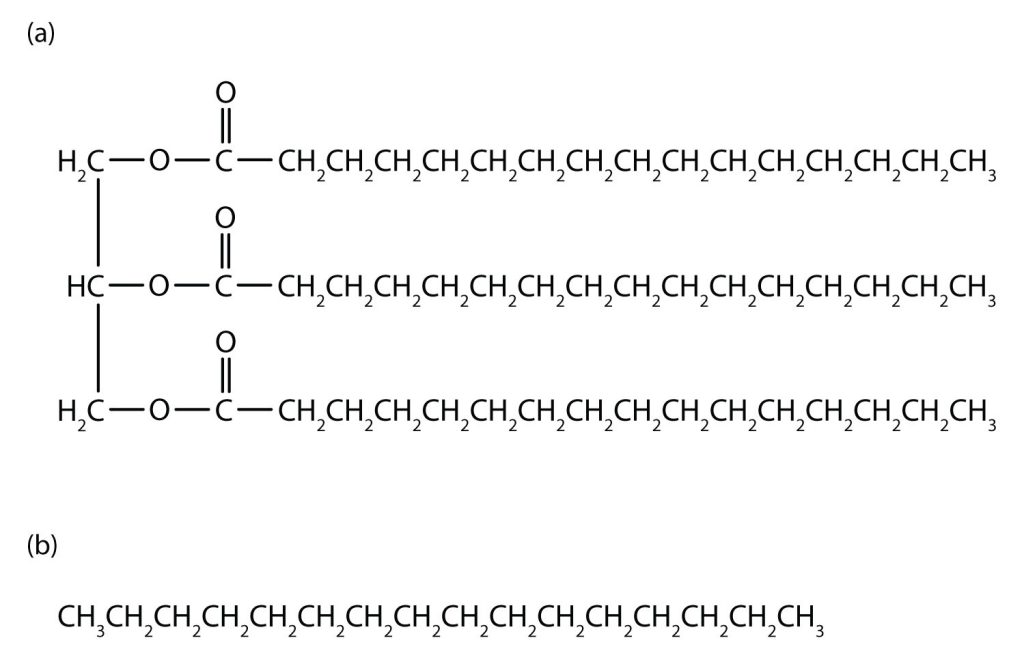
The three simplest alkanes—methane (CH4), ethane (C2H6), and propane (C3H8) shown in Figure 20.0a., are the beginning of a series of compounds in which any two members in a sequence differ by one carbon atom and two hydrogen atoms—namely, a CH2 unit (called methylene). Alkanes follow the general formula: CnH2n+2. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms. For example, an alkane with eight carbon atoms has the molecular formula C8H(2 × 8) + 2 = C8H18.
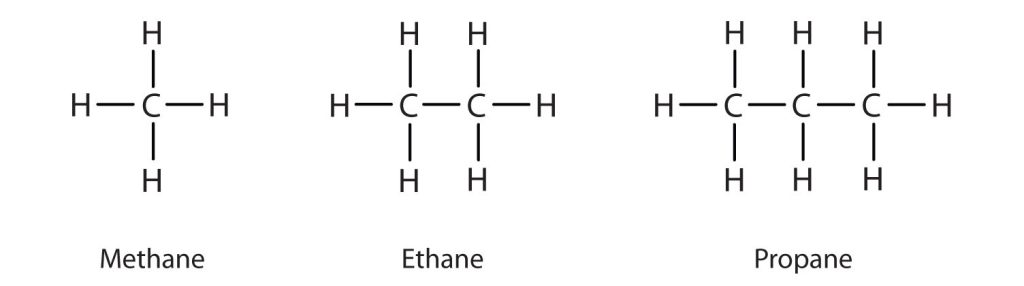
An Alkane Basis for Properties of Other Compounds
An understanding of the physical properties of the alkanes is important in that petroleum and natural gas and the many products derived from them—gasoline, bottled gas, solvents, plastics, and more—are composed primarily of alkanes. This understanding is also vital because it is the basis for describing the properties of other organic and biological compound families. For example, large portions of the structures of lipids consist of nonpolar alkyl groups as shown in Figure 20.0b. Lipids include the dietary fats and fatlike compounds called phospholipids and sphingolipids that serve as structural components of living tissues. These compounds have both polar and nonpolar groups, enabling them to bridge the gap between water-soluble and water-insoluble phases. This characteristic is essential for the selective permeability of cell membranes.