19.3 Aldehydes, Ketones, Carboxylic Acids, and Esters
Learning Objectives
By the end of this section, you will be able to:
- Classify aldehydes, ketones, carboxylic acids and esters
The Carbonyl Group
Another class of organic molecules contains a carbon atom connected to an oxygen atom by a double bond, commonly called a carbonyl group as shown in Figure 19.3a. The trigonal planar carbon in the carbonyl group can attach to two other substituents leading to several subfamilies (aldehydes, ketones, carboxylic acids and esters) described in this section.
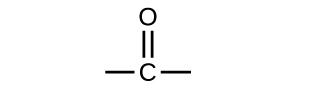
Aldehydes and Ketones
Both aldehydes and ketones contain a carbonyl group, a functional group with a carbon-oxygen double bond as shown in Figure 19.3a. The names for aldehyde and ketone compounds are derived using similar nomenclature rules as for alkanes and alcohols, and include the class-identifying suffixes -al and -one, respectively.
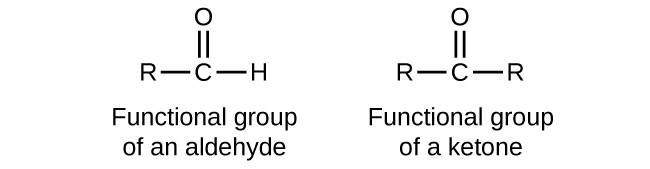
In an aldehyde, the carbonyl group is bonded to at least one hydrogen atom. In a ketone, the carbonyl group is bonded to two carbon atoms as shown in Figure 19.3b.
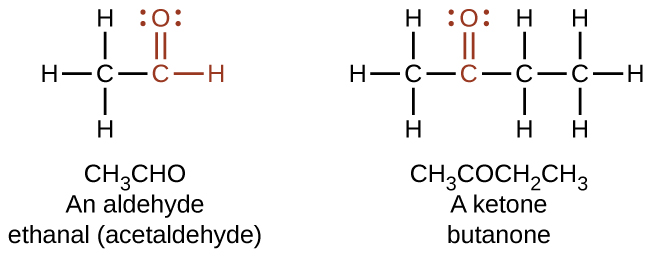
As text, an aldehyde group is represented as –CHO; a ketone is represented as –C(O)– or –CO– as shown in the examples within Figure 19.3c.
In both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal planar; the carbon atom exhibits sp2 hybridization. Two of the sp2 orbitals on the carbon atom in the carbonyl group are used to form σ bonds to the other carbon or hydrogen atoms in a molecule. The remaining sp2 hybrid orbital forms a σ bond to the oxygen atom. The unhybridized p orbital on the carbon atom in the carbonyl group overlaps a p orbital on the oxygen atom to form the π bond in the double bond.
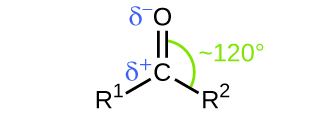
Like the [latex]\text{C} = \text{O}[/latex] bond in carbon dioxide, the [latex]\text{C} = \text{O}[/latex] bond of a carbonyl group is polar (recall that oxygen is significantly more electronegative than carbon, and the shared electrons are pulled toward the oxygen atom and away from the carbon atom). Many of the reactions of aldehydes and ketones start with the reaction between a Lewis base and the carbon atom at the positive end of the polar [latex]\text{C} = \text{O}[/latex] bond to yield an unstable intermediate that subsequently undergoes one or more structural rearrangements to form the final product (Figure 19.3d).
The importance of molecular structure in the reactivity of organic compounds is illustrated by the reactions that produce aldehydes and ketones. We can prepare a carbonyl group by oxidation of an alcohol—for organic molecules, oxidation of a carbon atom is said to occur when a carbon-hydrogen bond is replaced by a carbon-oxygen bond as shown in Figure 19.3e.
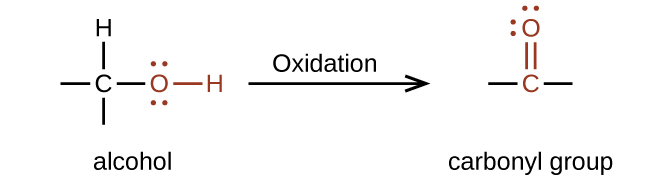
Formation of Aldehydes and Ketones
Aldehydes are commonly prepared by the oxidation of alcohols (Figure 19.3f), whose –OH functional group is located on the carbon atom at the end of the chain of carbon atoms in the alcohol:

Alcohols that have their –OH groups in the middle of the chain are necessary to synthesize a ketone (Figure 19.3g), which requires the carbonyl group to be bonded to two other carbon atoms:

An alcohol with its –OH group bonded to a carbon atom that is bonded to no or one other carbon atom will form an aldehyde. An alcohol with its –OH group attached to two other carbon atoms will form a ketone. If three carbons are attached to the carbon bonded to the –OH, the molecule will not have a C–H bond to be replaced, so it will not be susceptible to oxidation.
Formaldehyde, an aldehyde with the formula HCHO, is a colourless gas with a pungent and irritating odour. It is sold in an aqueous solution called formalin, which contains about 37% formaldehyde by weight. Formaldehyde causes coagulation of proteins, so it kills bacteria (and any other living organism) and stops many of the biological processes that cause tissue to decay. Thus, formaldehyde is used for preserving tissue specimens and embalming bodies. It is also used to sterilize soil or other materials. Formaldehyde is used in the manufacture of Bakelite, a hard plastic having high chemical and electrical resistance.
Dimethyl ketone, CH3COCH3, commonly called acetone, is the simplest ketone. It is made commercially by fermenting corn or molasses, or by oxidation of 2-propanol. Acetone is a colourless liquid. Among its many uses are as a solvent for lacquer (including fingernail polish), cellulose acetate, cellulose nitrate, acetylene, plastics, and varnishes; as a paint and varnish remover; and as a solvent in the manufacture of pharmaceuticals and chemicals.
Carboxylic Acids and Esters
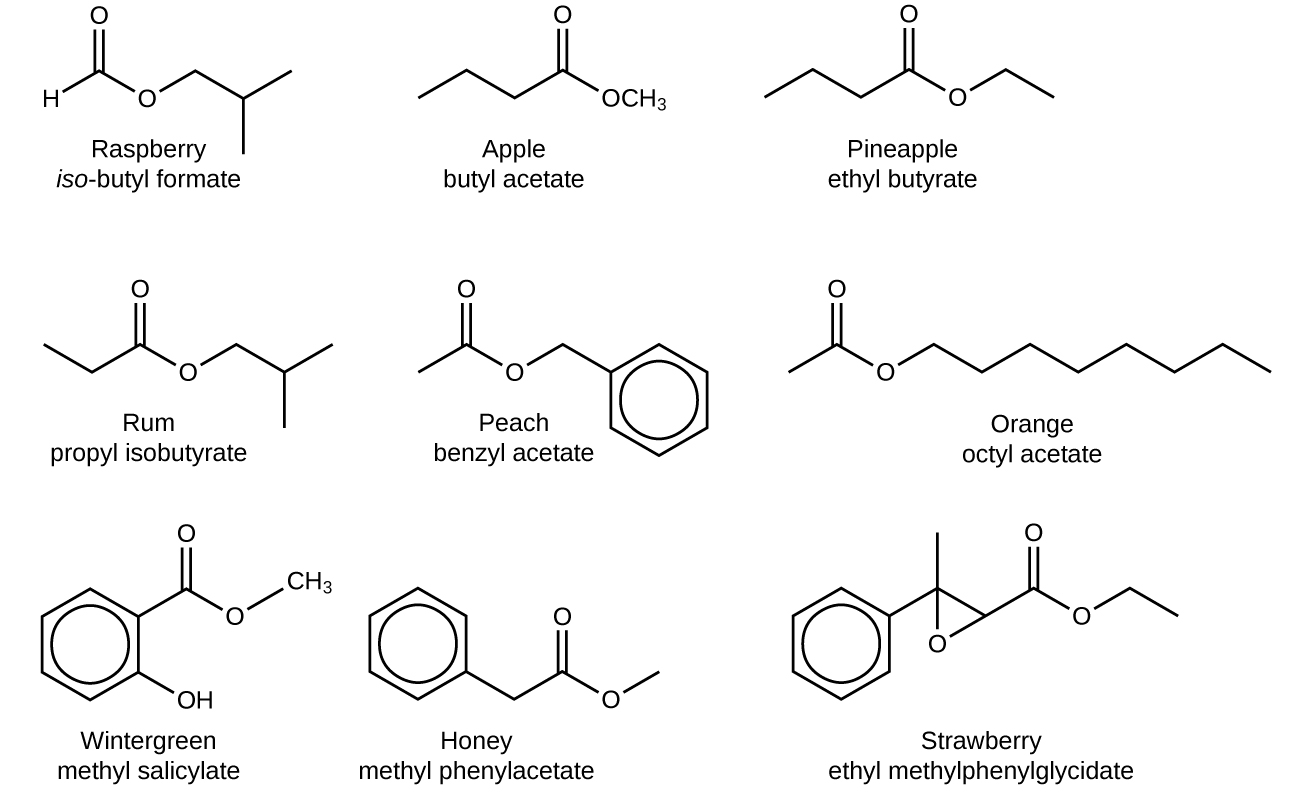
The odour of vinegar is caused by the presence of acetic acid, a carboxylic acid, in the vinegar. The odour of ripe bananas and many other fruits is due to the presence of esters, compounds that can be prepared by the reaction of a carboxylic acid with an alcohol. Because esters do not have hydrogen bonds between molecules, they have lower vapor pressures than the alcohols and carboxylic acids from which they are derived (see Figure 19.3h).
Both carboxylic acids and esters contain a carbonyl group with a second oxygen atom bonded to the carbon atom in the carbonyl group by a single bond (Figure 19.3i). In a carboxylic acid, the second oxygen atom also bonds to a hydrogen atom. In an ester, the second oxygen atom bonds to another carbon atom. The names for carboxylic acids and esters include prefixes that denote the lengths of the carbon chains in the molecules and are derived following nomenclature rules similar to those for inorganic acids and salts (see Figure 19.3i).
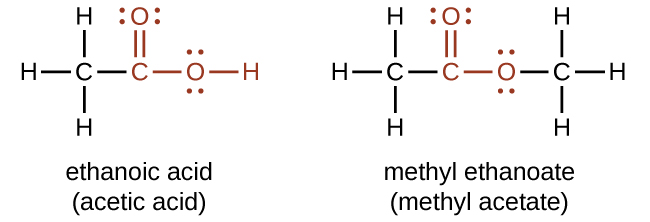
Carboxylic acids are weak acids, meaning they are not 100% ionized in water. Generally only about 1% of the molecules of a carboxylic acid dissolved in water are ionized at any given time. The remaining molecules are undissociated in solution.
The simplest carboxylic acid is formic acid, HCO2H, known since 1670. Its name comes from the Latin word formicus, which means “ant”; it was first isolated by the distillation of red ants. It is partially responsible for the pain and irritation of ant and wasp stings, and is responsible for a characteristic odour of ants that can be sometimes detected in their nests.
Acetic acid, CH3CO2H, constitutes 3–6% vinegar. Cider vinegar is produced by allowing apple juice to ferment without oxygen present. Yeast cells present in the juice carry out the fermentation reactions. The fermentation reactions change the sugar present in the juice to ethanol, then to acetic acid. Pure acetic acid has a penetrating odour and produces painful burns. It is an excellent solvent for many organic and some inorganic compounds, and it is essential in the production of cellulose acetate, a component of many synthetic fibers such as rayon.
The distinctive and attractive odours and flavours of many flowers, perfumes, and ripe fruits are due to the presence of one or more esters. Among the most important of the natural esters are fats (such as lard, tallow, and butter) and oils (such as linseed, cottonseed, and olive oils), which are esters of the trihydroxyl alcohol glycerine, C3H5(OH)3, with large carboxylic acids, such as palmitic acid, CH3(CH2)14CO2H, stearic acid, CH3(CH2)16CO2H, and oleic acid, CH3(CH2)7CH=CH(CH2)7CO2H. Oleic acid is an unsaturated acid; it contains a C=C double bond. Palmitic and stearic acids are saturated acids that contain no double or triple bonds.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from “18.3 Aldehydes, Ketones, Carboxylic Acids, and Esters” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
carbon atom double bonded to an oxygen atom
organic compound containing a carbonyl group bonded to two hydrogen atoms or a hydrogen atom and a carbon substituent
organic compound containing a carbonyl group with two carbon substituents attached to it
An organic compound that has a carboxyl functional group.
organic compound containing a carbonyl group with an attached oxygen atom that is bonded to a carbon substituent

