27.8 Plastics and Recycling
Learning Objectives
By the end of this section, you will be able to:
- Identify the type of polymer associated with each recycling number.
- Describe some different plastic recycling processes.
Recyclable Plastics
Seven groups of plastic polymers, each with specific properties, are used worldwide for packaging applications (see Table 27.8a.). Each group of plastic polymers can be identified by its plastic identification code (PIC), usually a number or a letter abbreviation (Figure 27.8a.). For instance, low-density polyethylene can be identified by the number “4” or the letters “LDPE”. The PIC appears inside a three-chasing-arrow recycling symbol. The symbol is used to indicate whether the plastic can be recycled into new products. These symbols can vary with producer and country.
The PIC was introduced by the Society of the Plastics Industry, Inc., to provide a uniform system for the identification of various polymer types and to help recycling companies separate various plastics for reprocessing. Manufacturers of plastic products are required to use PIC labels in some countries/regions and can voluntarily mark their products with the PIC where there are no requirements. Consumers can identify the plastic types based on the codes usually found at the base or at the side of the plastic products, including food/chemical packaging and containers. Not all categories are accepted by all local recycling authorities, so residents need to be informed about which kinds should be placed in recycling containers and which should be combined with ordinary trash.
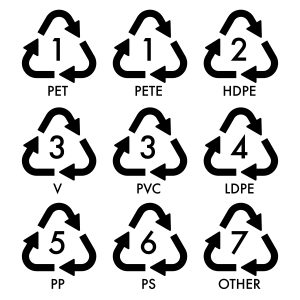
| Plastic identification code | Type of plastic polymer | Monomer | Properties | Common packaging applications | Melting temperatures (°C) |
|---|---|---|---|---|---|
 |
Polyethylene terephthalate (PET, PETE) |
|
Clarity, strength, toughness, barrier to gas and moisture. | Soft drink, water and salad dressing bottles; peanut butter and jam jars; ice cream cone lids; small consumer electronics | 250 |
|
|
High-density polyethylene (HDPE)
|
|
Stiffness, strength, toughness, resistance to moisture, permeability to gas | Water pipes, Gas & Fire Pipelines, Electrical & Communications conduit, hula hoop rings, five-gallon buckets, milk, juice and water bottles; grocery bags, some shampoo/toiletry bottles | 130 |
 |
Polyvinyl chloride (PVC) |
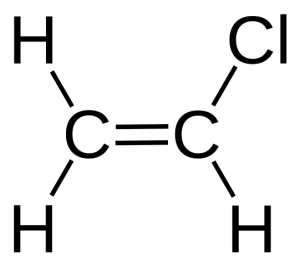 |
Versatility, ease of blending, strength, toughness. | Blister packaging for non-food items; cling films for non-food use. May be used for food packaging with the addition of the plasticizers needed to make natively rigid PVC flexible. Non-packaging uses are electrical cable insulation; rigid piping; vinyl records. | 240 |
 |
Low-density polyethylene (LDPE)
|
|
Ease of processing, strength, toughness, flexibility, ease of sealing, barrier to moisture | Frozen food bags; squeezable bottles, e.g., honey, mustard; cling films; flexible container lids | 120 |
|
Polypropylene (PP) |
|
Strength, toughness, resistance to heat, chemicals, grease and oil, versatile, barrier to moisture. | Reusable microwaveable ware; kitchenware; yogurt containers; margarine tubs; microwaveable disposable take-away containers; disposable cups; soft drink bottle caps; plates. | 173 |
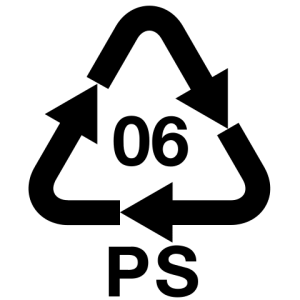 |
Polystyrene (PS) | styrene (Ethenylbenzene)
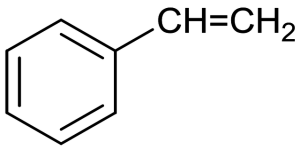 |
Versatility, clarity, easily formed | Egg cartons; packing peanuts; disposable cups, plates, trays and cutlery; disposable take-away containers | 240 |
|
Other (often polycarbonate or ABS) | various | Dependent on polymers or combination of polymers | Beverage bottles, baby milk bottles. Non-packaging uses for polycarbonate, compact discs, “unbreakable” glazing, electronic apparatus housing, lenses (including sunglasses), prescription glasses, automotive headlamps, riot shields, instrument panels. |
n/a |
Source: Except where otherwise noted, this table is adapted from “Plastic recycling” and “Resin identification code” on Wikipedia, licensed under CC BY-SA 4.0; edited by Samantha Sullivan Sauer with reference to Hein et al (2014). Except where otherwise noted, all images are in the Public Domain. Image credits for plastic identification code in column 1: 06-PS Image by Anton Poliakov, CC BY-SA 4.0; 07-O Image by Tomia, CC BY 2.5.
Often, products may be marked with a recycling symbol with number 7 or other numbers. These plastics are not standardized and as such are not recyclable in municipal process. The specific polymer is sometimes indicated but not always resulting in mixed plastics.
To see a summary of various plastics and those that are recyclable, refer to Figure 27.8b.

Watch What Numbers of Plastic are Recyclable? – YouTube (2 mins)
Video source: Quality Logo Products. (2017, October 16). What Numbers of Plastic are Recyclable? – YouTube [Video]. YouTube.
Recycling Process
Because plastics are largely non-biodegradable, the huge quantity (one estimate is 108 metric tons per year) of plastic materials produced for consumer and industrial use has created a gigantic problem of what to do with plastic waste. Plastics are difficult to incinerate safely and threaten to overwhelm the capacity of landfills (Figure 27.8c). An additional consideration is that production of most of the major polymers consumes non-renewable hydrocarbon resources.

Plastics recycling has become a major industry, greatly aided by enlightened trash management policies in the major developed nations. However, it is plagued with some special problems of its own:
- Recycling is only profitable when there is a market for the regenerated material. Such markets vary with the economic cycle. They practically disappeared during the recession that commenced in 2008.
- The energy-related costs of collecting and transporting plastic waste, and especially of processing it for re-use, are frequently the deciding factor in assessing the practicability of recycling.
- Collection of plastic wastes from diverse sources and locations and their transport to processing centers consumes energy and presents numerous operational problems.
- Most recycling processes are optimized for particular classes of polymers. The diversity of plastic types necessitates their separation into different waste streams — usually requiring manual (i.e., low-cost) labor. This in turn encourages shipment of these wastes to low-wage countries, thus reducing the availability of recycled materials in the countries in which the plastics originated.
In general, plastics are collected and transported to a facility where they are sorted by recycling code. This is one of the major limiting factors of the process. There are several ways the plastics are separated including by hand, through physical property comparison and using technology. The plastics are then shredded, washed, and granulated into pellets. These pellets are then transported and reformed into future products. Products using recycled pellets or fibers will have different properties than the original plastic. In addition, there is a limit to the number of times plastics can be recycled and reformed resulting in waste production. (Hein et al., 2014; Brunning, 2018)
Links to Enhanced Learning
To read more about how plastics are recycled, see this infographic from Chemical & Engineering News, Periodic graphics: How is plastic recycled?.
Some of the major recycling processes include:
- Thermoplastic polymers can be melted and pelletized, but those of widely differing types must be treated separately to avoid incompatibility problems.
- Thermosetting polymers are usually shredded and used as filler material in recycled thermoplastic polymers.
- Thermal decomposition processes that can accommodate mixed kinds of plastics and render them into fuel oil, but the large inputs of energy they require have been a problem. Read more about Converting waste plastic bottles into jet fuel on the Chemistry World website
- A small number of condensation polymers can be depolymerized so that the monomers can be recovered and re-used.
- A process to recycle scrap steel has been developed in which many kinds of plastic can be used as a carbon source.
- Mixed recycling of different plastics is possible. It does not require their separation and is called compatibilization. It requires use of special chemical bridging agents compatibilizers. Compatibilization can help to keep the quality of recycled material and to skip often expensive and inefficient preliminary scanning of waste plastics streams and their separation/purification.
- Re-use of rubber tires in the construction industry is one method to support the increasing reluctance of landfills to accept used tires.
Attribution & References
Except where otherwise noted, this page has been adapted by Samantha Sullivan Sauer from
- “10.7: Plastics and the Environment” In Map: Chemistry for Changing Times (Hill and McCreary) by Marisa Alviar-Agnew (Sacramento City College) & LibreTexts, licensed under CC BY-NC-SA 4.0. Contributors from original source:
- Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook
- Marisa Alviar-Agnew (Sacramento City College)
- Wikipedia
References cited in text:
Hein, M., Pattison, S., Arena, S., & Best, L. R. (2014). Introduction to General, Organic, and Biochemistry (11th ed.). Wiley.
Brunning, A. (2018, April 20). Periodic Graphics: How is plastic recycled?. Chemical & Engineering News.


