29.3 Chromatographic Columns
Learning Objectives
By the end of this section, you will be able to:
- Describe the purpose and procedure of column chromatography.
The same principles used in thin layer chromatography and paper chromatography can be applied on a larger scale to separate mixtures in column chromatography. Column chromatography is often used to purify compounds made in the lab.
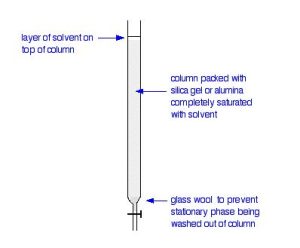
In thin layer chromatography, the stationary phase is a thin layer of silica gel or alumina on a glass, metal or plastic plate. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column (Figure 29.3a.). Various sizes of chromatography columns are used.
Separating Mixtures with Columns
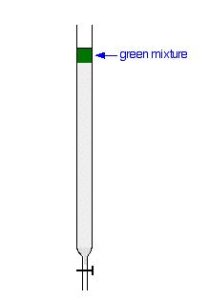
To separate a green mixture of two-coloured compounds, one yellow, one blue, it starts with a concentrated solution of the mixture preferably in the solvent used in the column. First, open the tap to allow the solvent already in the column to drain so that it is level with the top of the packing material, and then add the solution carefully to the top of the column. Then, open the tap again so that the coloured mixture is all absorbed into the top of the packing material, so that it might look like Figure 29.3b.
Next, add fresh solvent to the top of the column, trying to disturb the packing material as little as possible. Then, open the tap so that the solvent can flow down through the column, collecting it in a beaker or flask at the bottom. As the solvent runs through, keep adding fresh solvent to the top so that the column never dries out. Figure 29.3c. shows what might happen over time.
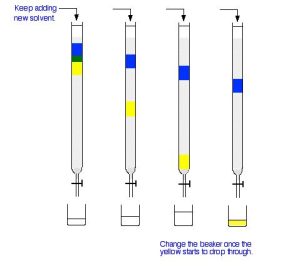
In Figure 29.3c., the blue compound is moving slower than the yellow compound through the column. This means, in this setup, the blue compound is more polar than the yellow one. It must adsorb more strongly to the silica gel or alumina than the yellow one. The less polar yellow one spends more of its time in the solvent and therefore washes through the column much faster. The process of washing a compound through a column using a solvent is known as elution. The solvent is sometimes known as the eluent.
To collect the blue compound, continuing with the same solvent is going to take a lot of time and solvent because it is travelling so slowly. However, there is no reason why the solvent can’t be change during elution. By replacing the solvent with a more polar one once the yellow has all been collected, will speed the blue compound through the column. With a more polar solvent, the blue compound spends more time in solution, and so moves faster. By using two different solvents, decent separation of the components is possible as well as keeping waste and time to a minimum.
TLC can be combined with column chromatography if the mixture is colourless by collecting the output in separate containers and testing each with a TLC process.
Instruments that use Chromatographic Columns
Chromatographic columns are part of the instrumentation that is used in chromatography. Several chromatographic methods that use columns are gas chromatography (GC), liquid chromatography (LC), Ion exchange chromatography (IEC), and size exclusion chromatography (SEC). The basic principles of chromatography can be applied to all five methods.
In gas chromatography (GC), the mobile phase is a gas. Gas chromatographic columns are usually between 1 and 100 meters long. The liquid stationary phase is bonded or adsorbed onto the surface of an open tubular (capillary) column, or onto a packed solid support inside the column. Matching the polarities of the analyte and stationary phase is not an exact science. The two should have similar polarities. The thickness of the stationary phase ranges between 0.1 and 8 µm. The thicker the layer the more volatile the analyte can be.
High performance liquid chromatography (HPLC) is a type of liquid chromatography that uses a liquid mobile phase. The same basic principles from gas chromatography are applied to liquid chromatography. There are three basic types of liquid chromatographic columns: liquid-liquid, liquid-solid, and ion-exchange. Liquid-liquid chromatographic columns have the liquid stationary phase bonded or absorbed to the surface of the column, or packed material. liquid-liquid chromatographic columns are not as popular because they have limited stability, and they are inconvenient. Partitioning occurs between the two different liquids of the mobile and stationary phases. In liquid-solid chromatographic columns the stationary phase is a solid and the analyte absorbs onto the stationary phase which separates the components of the mixture. In ion-exchange chromatographic columns the stationary phase is an ion-exchange resin and partitioning occurs with ion exchanges that occur between the analyte and stationary phase. The most common HPLC columns are made from stainless steel, but they can be also made out of thick glass, polymers, a combination of stainless steel and glass, or a combination of stainless steel and polymers. Typical HPLC analytical columns are between 3 and 25 cm long and have a diameter of 1 to 5 mm. The columns are usually straight unlike GC columns. Particles that pack the columns have a typical diameter between 3 to 5 µm. Liquid chromatographic columns will increase in efficiency when the diameter of the packed particles inside the column decreases.
Ion exchange chromatographic (IEC) columns are used to separate ions and molecules that can be easily ionized. Separation of the ions depends on the ion’s affinity for the stationary phase, which creates an ion exchange system. The electrostatic interactions between the analytes, mobile phase, and the stationary phase, contribute to the separation of ions in the sample. Only positively or negatively charged complexes can interact with their respective cation or anion exchangers. Common packing materials for ion exchange columns are amines, sulfonic acid, diatomaceous earth, styrene-divinylbenzene, and cross-linked polystyrene resins.
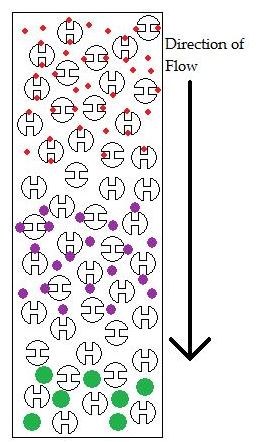
Size exclusion chromatographic (SEC) columns separate molecules based upon their size, not molecular weight. A common packing material for these columns is molecular sieves. The molecular sieves have pores that small molecules can go into, but large molecules cannot (Figure 29.3d.). This allows the larger molecules to pass through the column faster than the smaller ones. Other packing materials for size exclusion chromatographic columns are polysaccharides and other polymers, and silica. The pore size for size exclusion separations varies between 4 and 200 nm.