22.4 Aromatic Compounds – Structure and Naming
Learning Objectives
By the end of this section, you will be able to:
- Describe the bonding in benzene and the way typical reactions of benzene differ from those of the alkenes.
- Recognize aromatic compounds from structural formulas.
- Name aromatic compounds given formulas.
- Write formulas for aromatic compounds given their names.
Next, we consider a class of hydrocarbons with molecular formulas like those of unsaturated hydrocarbons, but which, unlike the alkenes, do not readily undergo addition reactions. These compounds comprise a distinct class, called aromatic hydrocarbons, with unique structures and properties. Historically, benzene-like substances were called aromatic hydrocarbons because they had distinctive aromas. Today, an aromatic compound is any compound that contains a benzene ring or has certain benzene-like properties (but not necessarily a strong aroma). You can recognize the aromatic compounds in this text by the presence of one or more benzene rings in their structure. Some representative aromatic compounds and their uses are listed in Table 22.4a., where the benzene ring is represented as C6H5.
The simplest of these compounds. Benzene (C6H6) is of great commercial importance, but it also has noteworthy health effects.
The formula C6H6 seems to indicate that benzene has a high degree of unsaturation. (Hexane, the saturated hydrocarbon with six carbon atoms has the formula C6H14—eight more hydrogen atoms than benzene.) However, despite the seeming low level of saturation, benzene is rather unreactive. It does not, for example, react readily with bromine, which, is a test for unsaturation.
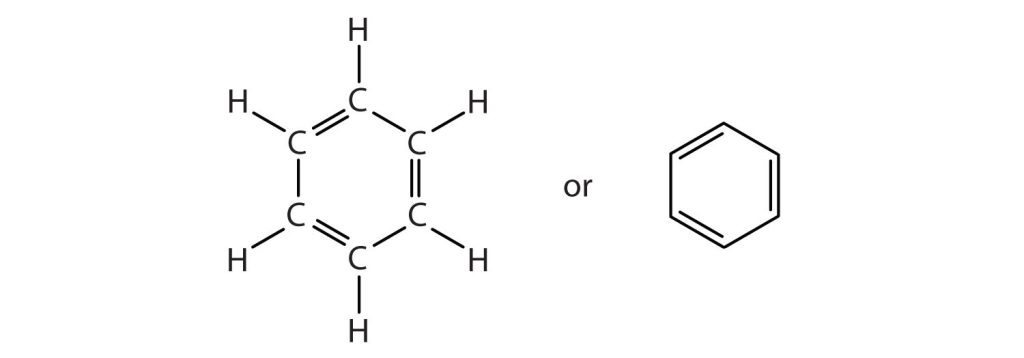
To explain the surprising properties of benzene, chemists suppose the molecule has a cyclic, hexagonal, planar structure of six carbon atoms with one hydrogen atom bonded to each. We can write a structure with alternate single and double bonds, either as a full structural formula or as a line formula as shown in Figure 22.4a.
However, these structures do not explain the unique properties of benzene. Furthermore, experimental evidence indicates that all the carbon-to-carbon bonds in benzene are equivalent, and the molecule is unusually stable. Chemists often represent benzene as a hexagon with an inscribed circle as in Figure 22.4b.
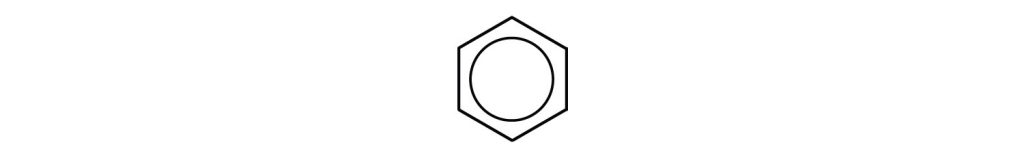
The inner circle indicates that the valence electrons are shared equally by all six carbon atoms (that is, the electrons are delocalized, or spread out, over all the carbon atoms). It is understood that each corner of the hexagon is occupied by one carbon atom, and each carbon atom has one hydrogen atom attached to it. Any other atom or groups of atoms substituted for a hydrogen atom must be shown bonded to a particular corner of the hexagon. We use this modern symbolism, but many scientists still use the earlier structure with alternate double and single bonds.
| Name | Structure | Typical Uses |
|---|---|---|
| aniline | C6H5–NH2 | starting material for the synthesis of dyes, drugs, resins, varnishes, perfumes; solvent; vulcanizing rubber |
| benzoic acid | C6H5–COOH | food preservative; starting material for the synthesis of dyes and other organic compounds; curing of tobacco |
| bromobenzene | C6H5–Br | starting material for the synthesis of many other aromatic compounds; solvent; motor oil additive |
| nitrobenzene | C6H5–NO2 | starting material for the synthesis of aniline; solvent for cellulose nitrate; in soaps and shoe polish |
| phenol | C6H5–OH | disinfectant; starting material for the synthesis of resins, drugs, and other organic compounds |
| toluene | C6H5–CH3 | solvent; gasoline octane booster; starting material for the synthesis of benzoic acid, |
Table source: “13.8: Structure and Nomenclature of Aromatic Compounds” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
Many aromatic based compounds have pleasant odours but they are generally toxic with some being carcinogenic. Users should avoid inhaling any vapours. Lighter weight aromatic hydrocarbons are highly flammable and have a sooty flame. Like all hydrocarbons, aromatics are less dense than water and not soluble in water. Their boiling points tend to increase in molar mass, but their melting points are independent on molar mass. Symmetry in the molecule leads to much higher melting points. (Roberts & Caserio, 1977).
Spotlight on Everyday Chemistry: August Kekule and The Benzene Structure
August Kekule was the first one to depict the benzene structure as a ring-like structure with alternating single and double bonds. For more information refer to infographic 22.4a. below.
Example 22.4a – Identifying Aromatic Compounds
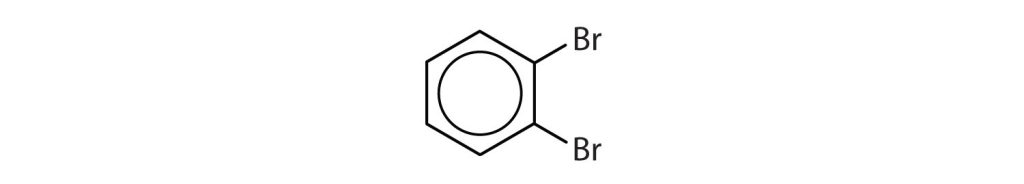
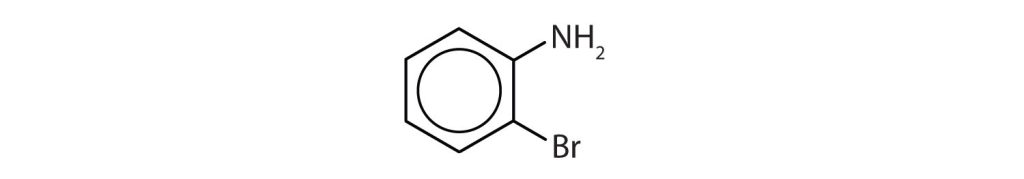
Which compounds are aromatic?
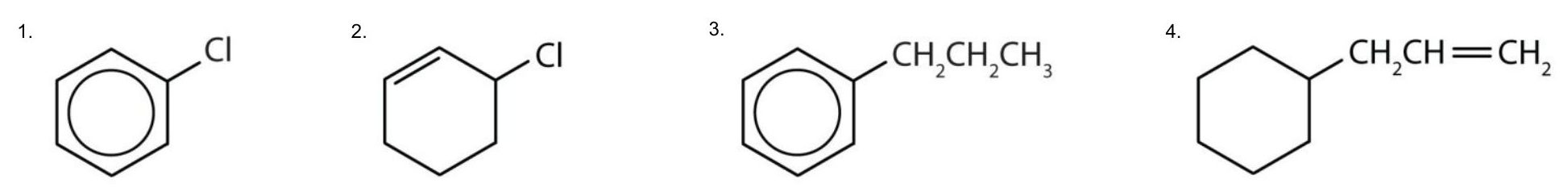
Solution
- The compound has a benzene ring (with a chlorine atom substituted for one of the hydrogen atoms); it is aromatic.
- The compound is cyclic, but it does not have a benzene ring; it is not aromatic.
- The compound has a benzene ring (with a propyl group substituted for one of the hydrogen atoms); it is aromatic.
- The compound is cyclic, but it does not have a benzene ring; it is not aromatic.
Spotlight on Everyday Chemistry: Benzene Health Hazards and Connection to Common Medical Ingredients
Benzene is a liquid that smells like gasoline, boils at 80°C, and freezes at 5.5°C. Most of the benzene used commercially comes from petroleum. It is employed as a starting material for the production of detergents, drugs, dyes, insecticides, and plastics. It was formerly used to decaffeinate coffee and was a significant component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. It was removed from many product formulations in the 1950s, but others continued to use benzene in products until the 1970s when it was associated with leukemia deaths. Benzene is still important in industry as a precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent for such things as cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is now known to have both short- and long-term toxic effects. The inhalation of large concentrations can cause nausea and even death due to respiratory or heart failure, while repeated exposure leads to a progressive disease in which the ability of the bone marrow to make new blood cells is eventually destroyed. This results in a condition called aplastic anemia, in which there is a decrease in the numbers of both the red and white blood cells.
Though benzene alone has been shown to potentially affect your health, substances containing the benzene ring are common in both animals and plants. Plants can synthesize the benzene ring from carbon dioxide, water, and inorganic materials. Animals cannot synthesize it, but they are dependent on certain aromatic compounds for survival and therefore must obtain them from food. Phenylalanine, tyrosine, and tryptophan (essential amino acids) and vitamins K, B2 (riboflavin), and B9 (folic acid) all contain the benzene ring. Many important drugs, a few of which are shown in Table 22.4b., also feature a benzene ring.
| Name | Structure |
|---|---|
| aspirin | 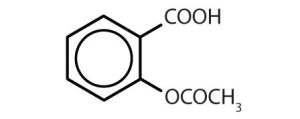 |
| acetaminophen | 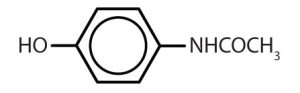 |
| ibuprofen | 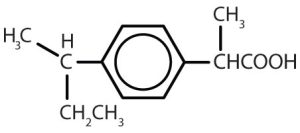 |
| amphetamine | 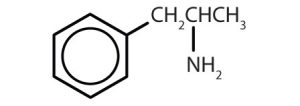 |
| sulfanilamide | 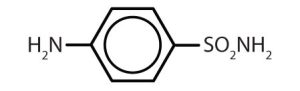 |
Naming Aromatic Compounds
Naming Monosubstituted Benzenes
In the International Union of Pure and Applied Chemistry (IUPAC) system, aromatic hydrocarbons are named as derivatives of benzene. Figure 22.4c. shows four examples. In these structures, it is immaterial whether the single substituent is written at the top, side, or bottom of the ring: a hexagon is symmetrical, and therefore all positions are equivalent.
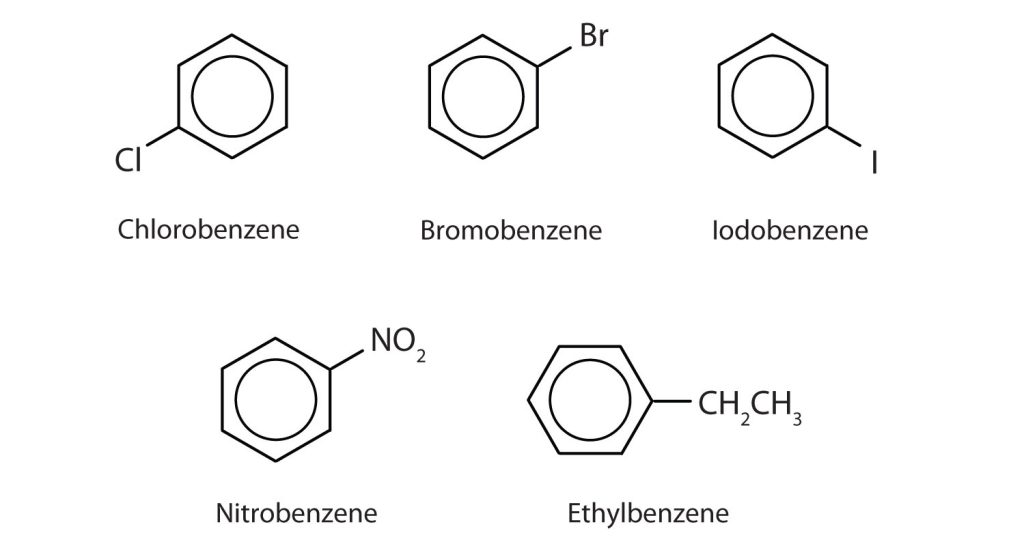
Although some compounds are referred to exclusively by IUPAC names, some are more frequently denoted by common names, as is indicated in Table 22.4a. and shown in Figure 22.4d.
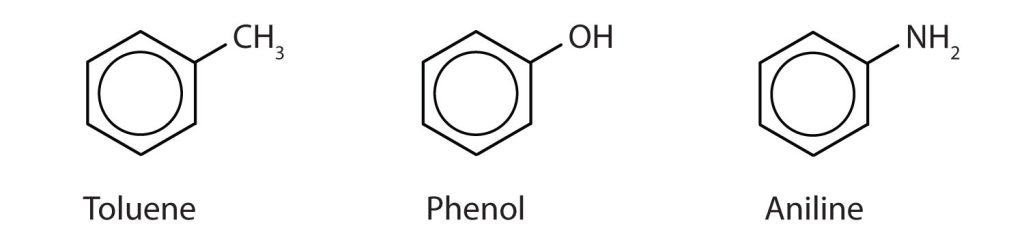
Naming Polysubstituted Benzenes
When there is more than one substituent, the corners of the hexagon are no longer equivalent, so we must designate the relative positions. There are three possible disubstituted benzenes, and we can use numbers to distinguish them (Figure 22.4e.). We start numbering at the carbon atom to which one of the groups is attached and count toward the carbon atom that bears the other substituent group by the shortest path.
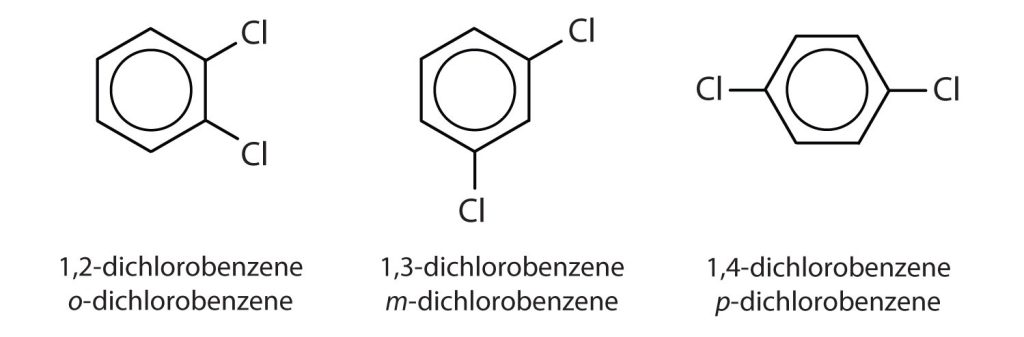
The structural differences in the three dichlorobenzene compounds in Figure 22.4e. lead to differences in physical properties. Table 22.4c. highlights some of the key physical properties of dichlorobenzenes.
| Compound | Chemical Formula | Molecular Weight | Boiling Point | Melting Point |
|---|---|---|---|---|
| 1,2-dichlorobenzene
(o-dichlorobenzene) |
C6H4Cl2 | 147.00 g/mol | 180oC | -17oC |
| 1,3-dichlorobenzene
(m-dichlorobenzene) |
C6H4Cl2 | 147.00 g/mol | 173oC | -25oC |
| 1,4-dichlorobenzene
(p-dichlorobenzene) |
C6H4Cl2 | 147.00 g/mol | 174oC | 53oC |
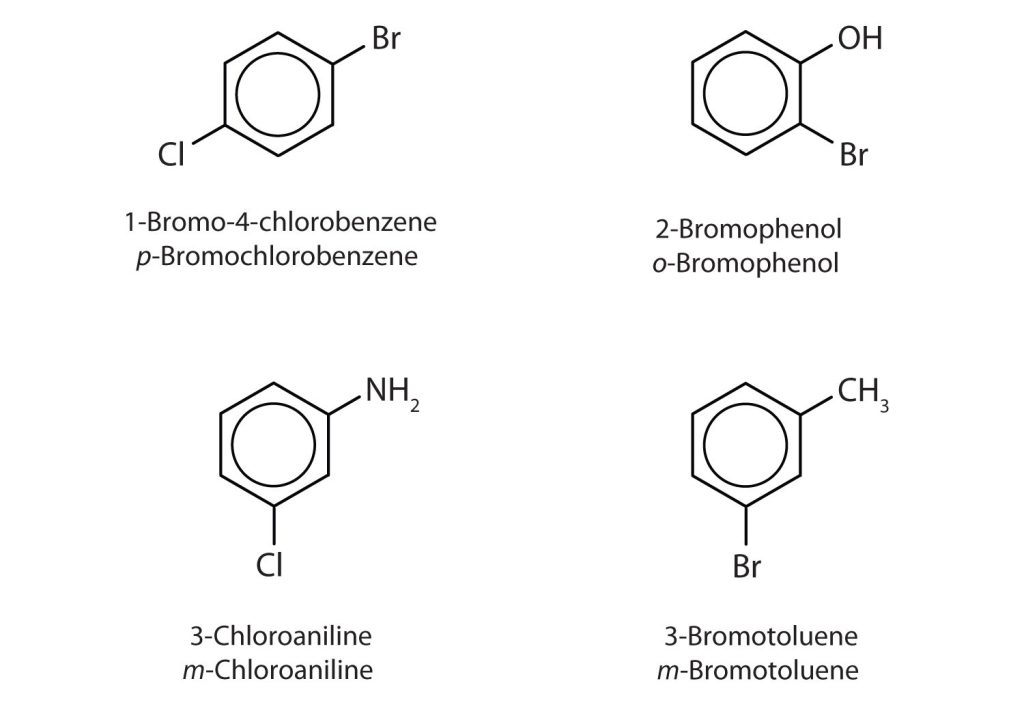
In Figure 22.4e., common names are also used: the prefix ortho (o-) for 1,2-disubstitution, meta (m-) for 1,3-disubstitution, and para (p-) for 1,4-disubstitution. The substituent names are listed in alphabetical order. The first substituent is given the lowest number. When a common name is used, the carbon atom that bears the group responsible for the name is given the number 1 as demonstrated in Figure 22.4f.
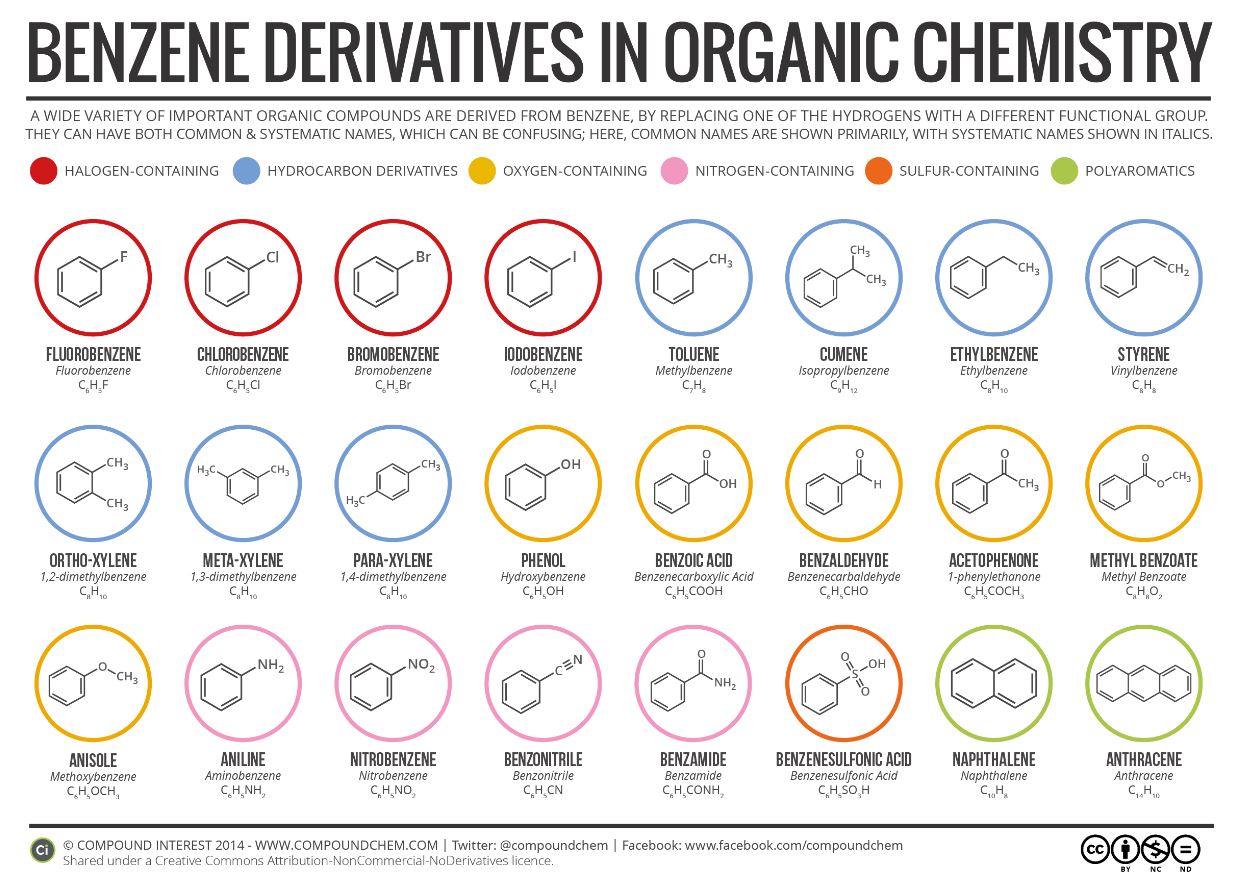
Infographic 22.4b. shows a section of benzene derivatives with their IUPAC and common names.
Example 22.4b
Name each compound using both the common name and the IUPAC name.
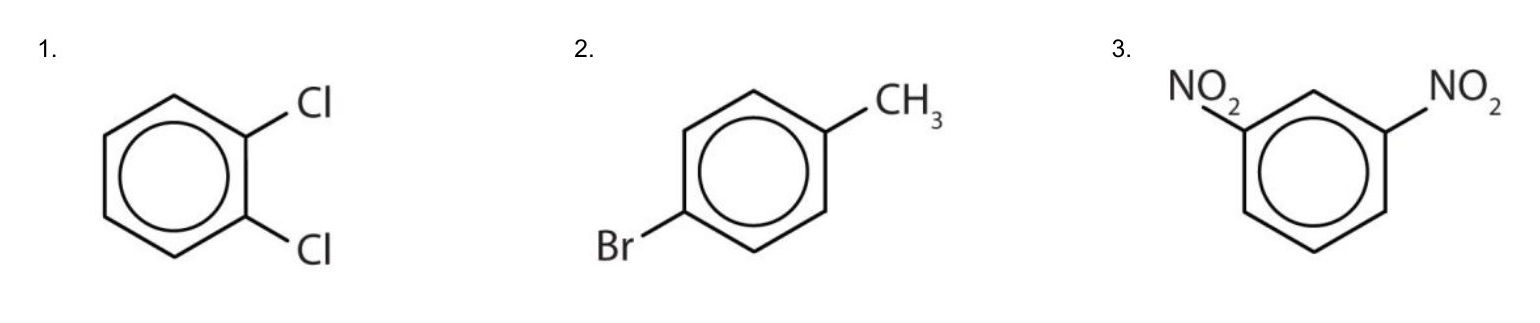
Solution
- The benzene ring has two chlorine atoms (dichloro) in the first and second positions. The compound is o-dichlorobenzene or 1,2-dichlorobenzene.
- The benzene ring has a methyl (CH3) group. The compound is therefore named as a derivative of toluene. The bromine atom is on the fourth carbon atom, counting from the methyl group. The compound is p-bromotoluene or 4-bromotoluene.
- The benzene ring has two nitro (NO2) groups in the first and third positions. It is m-dinitrobenzene or 1,3-dinitrobenzene.
Note: The nitro (NO2) group is a common substituent in aromatic compounds. Many nitro compounds are explosive, most notably 2,4,6-trinitrotoluene (TNT).
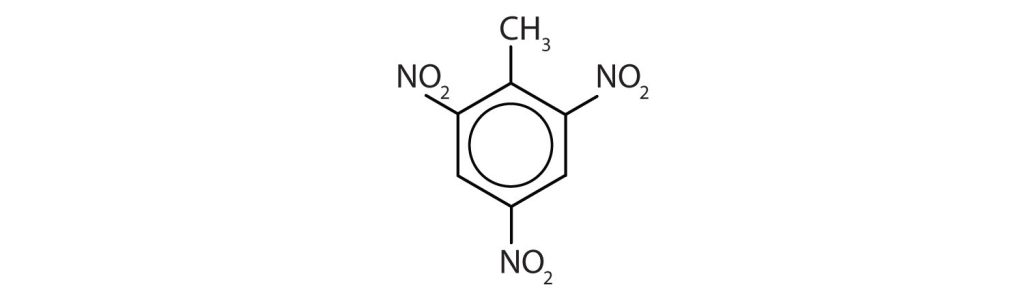
Exercise 22.4a
Name each compound using both the common name and the IUPAC name.
Naming Compounds with an Aromatic Substituent
Sometimes an aromatic group is found as a substituent bonded to a nonaromatic entity or to another aromatic ring. The group of atoms remaining when a hydrogen atom is removed from an aromatic compound is called an aryl group. The most common aryl group is derived from benzene (C6H6) by removing one hydrogen atom (C6H5) and is called a phenyl group (Figure 22.4g.), from pheno, an old name for benzene.
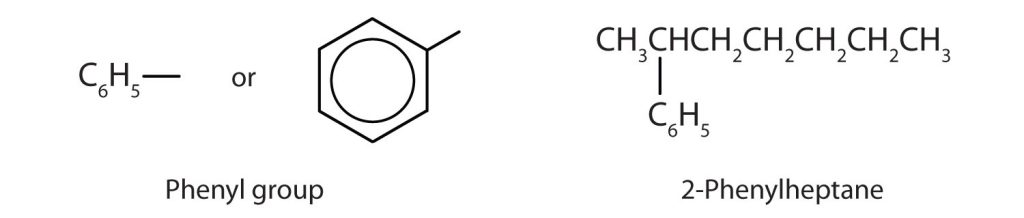
Exercise 22.4b
Name this compound.
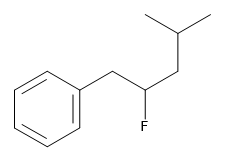
Check Your Answer:[2]
Activity source: Exercise 22.4b is created by Samantha Sullivan Sauer, using images from Biovia Draw, licensed under CC BY-NC 4.0
Spotlight on Everyday Chemistry: Benzene Derivatives in Sunscreen and New Car Smell
A number of sunscreens that are UVA and UVB blockers are made with ingredients that contain benzene structures. For a detailed look at the various substituted benzene ingredients, see infographic 22.4c. below.

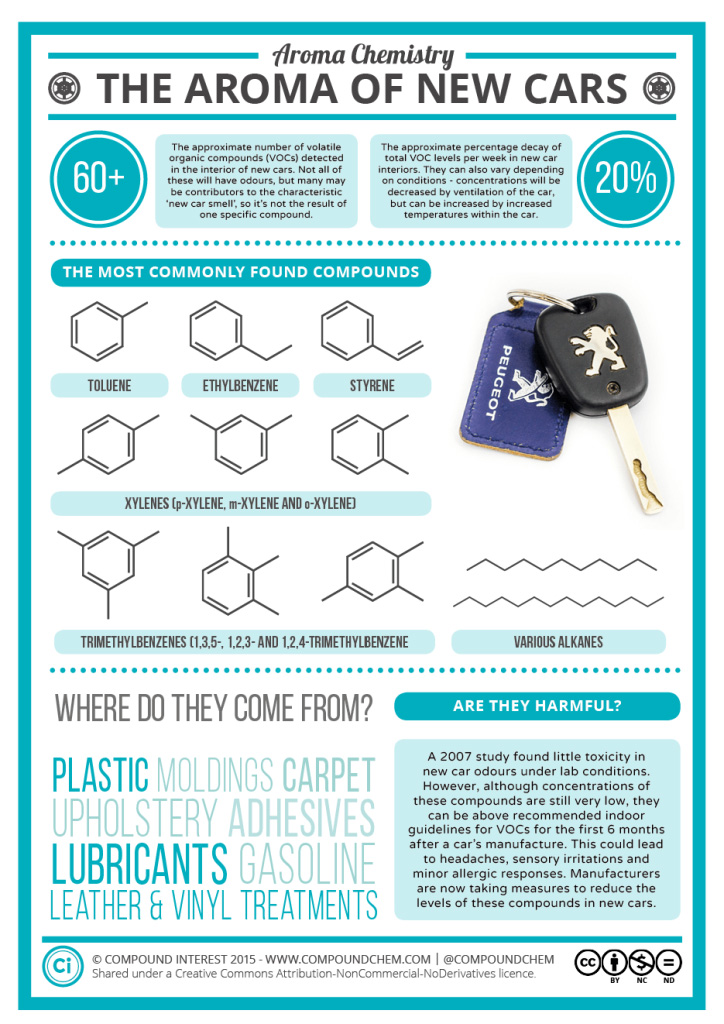
Not only are benzene compounds found in products we use such as sunscreen, but they can also be found in objects we handle. A new car for example will often have a “new car smell”. This smell is associated with numerous volatile organic compounds (VOCs) that are made up of compounds that contain a benzene ring. For more information about the types of VOCs found within a new car see Infographic 22.4d.
Polycyclic Aromatic Hydrocarbons
Some common aromatic hydrocarbons consist of fused benzene rings—rings that share a common side. These compounds are called polycyclic aromatic hydrocarbons (PAHs). A few examples are shown in Figure 22.4h.
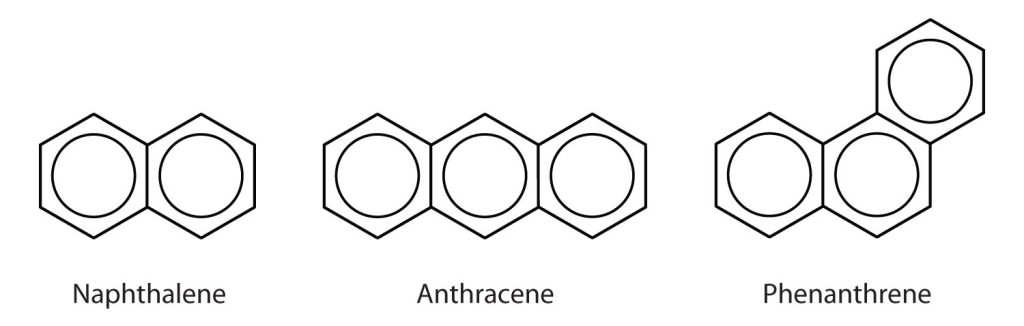
The three examples (naphthalene, anthracene and phenanthrene), shown here are colourless, crystalline solids generally obtained from coal tar. Naphthalene has a pungent odour and is used in mothballs. Anthracene is used in the manufacture of certain dyes. Steroids, a large group of naturally occurring substances, contain the phenanthrene structure.
Spotlight on Everyday Chemistry: Glow Sticks
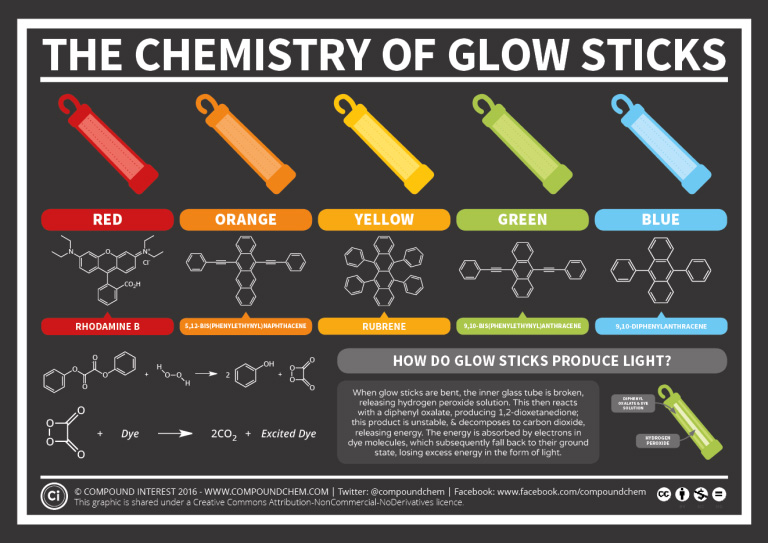
Glow sticks are effectively used at night so that you can be visible or to add some fun to an event. They come in all sorts of colours such as red, orange, yellow, green and blue. Benzene containing compounds are responsible for the various colours produced. Additionally, the reaction that allows the glow stick to glow, is from the reactions involving benzene containing compounds. Next time you use a glow stick, you can thank the benzene rings! For more information, see infographic 22.4e. below.
Attribution & References
Except where otherwise noted, this page is adapted by David Wegman, Adrienne Richards and Samantha Sullivan Sauer from “13.8: Aromatic Compounds and the Structure of Benzene” and “13.9: Naming Aromatic Compounds” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.) by LibreTexts, licensed under CC BY-NC-SA 3.0. / A derivative of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
References cited in-text
National Center for Biotechnology Information (2024a). PubChem Compound Summary for CID 7239, 1,2-Dichlorobenzene. Retrieved January 14, 2024. .
National Center for Biotechnology Information (2024b).PubChem Compound Summary for CID 10943, 1,3-Dichlorobenzene. Retrieved January 14, 2024.
National Center for Biotechnology Information (2024c). PubChem Compound Summary for CID 4685, 1,4-Dichlorobenzene. Retrieved January 14, 2024.
Roberts, J. D., & Caserio, M. C. (1977). Basic Principles of Organic Chemistry, (2nd ed.) W. A. Benjamin, Inc.
compounds containing a benzene ring