20.5 Halogenated Alkanes
Learning Objectives
By the end of this section, you will be able to:
- Name halogenated hydrocarbons given formulas and write formulas for these compounds given names.
Halogenated Alkanes
Many organic compounds are closely related to the alkanes. As we noted previously, alkanes react with halogens to produce halogenated hydrocarbons, the simplest of which have a single halogen atom substituted for a hydrogen atom of the alkane. Even more closely related are the cycloalkanes, compounds in which the carbon atoms are joined in a ring, or cyclic fashion.
Halogens are found in column 7A of the periodic table and include Fluorine, Chlorine, Bromine and Iodine. Refer to Appendix A: Key Element Information for more details about halogens.
Alkyl halides are encountered less frequently than their oxygen-containing relatives and are not often involved in the biochemical pathways of terrestrial organisms, but some of the kinds of reactions they undergo—nucleophilic substitutions and eliminations—are encountered frequently. Thus, alkyl halide chemistry is a relatively simple model for many mechanistically similar but structurally more complex reactions found in biomolecules.
Now that we’ve covered the chemistry of hydrocarbons, it’s time to start looking at more complex substances that contain elements in addition to C and H. We’ll begin by discussing the chemistry of organohalides, compounds that contain one or more halogen atoms.
Halogen-substituted organic compounds are widespread in nature, and more than 5000 organohalides have been found in algae and various other marine organisms. Chloromethane, for instance, is released in large amounts by ocean kelp, as well as by forest fires and volcanoes. Halogen-containing compounds also have an array of industrial applications, including their use as solvents, inhaled anesthetics in medicine, refrigerants, and pesticides as shown in Figure 20.5b.
Still other halo-substituted compounds are used as medicines and food additives. The nonnutritive sweetener sucralose, marketed as Splenda, contains three chlorine atoms, for instance. Sucralose is about 600 times as sweet as sucrose, so only 1 mg is equivalent to an entire teaspoon of table sugar. Refer to Figure 20.5c. for the structural formula of sucralose.
A large variety of organohalides are known. The halogen might be bonded to an alkynyl group (C≡C−X), a vinylic group (C═C–X), an aromatic ring (Ar−X), or an alkyl group.
The reactions of alkanes with halogens produce halogenated hydrocarbons, compounds in which one or more hydrogen atoms of a hydrocarbon have been replaced by halogen atoms (F, Cl, Br and I):

The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane) as shown in Figure 20.5d. above.
Indigenous Perspectives: The Impact of Fluorocarbons on Inuit People of Canada
The long-chained fluorocarbon ending with a -SO3H group is known as perfluorooctanesulfonic acid also known as PFOS (Figure 20.5e.). PFOS and their relatives contain a long fluorocarbon backbone that is extremely resistant to decomposition. In addition, the PFOS family of compounds are volatile and can spread throughout the Earth’s surface. Research has shown that through global distillation, these PFOS have accumulated in the Arctic. This is a problematic health concern for the Inuit in the north. Several food sources that are part of the Inuit diet have been tested and contain PFOS. Exposure to PFOS have numerous health implications such as cancer, endocrine delays and others as described in the article below. There is no end in sight from exposure to these harmful fluorocarbons as they are expected to remain in the arctic for hundreds and possibly thousands of years (Anderson & Rayner-Canham, 2022).
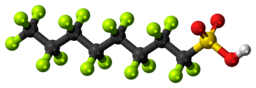
For more detailed information regarding the fluorocarbons and their impact on the Inuit see the following link: PFOS | Chem 13 News Magazine | University of Waterloo (uwaterloo.ca).
Naming Haloalkanes
Although commonly called alkyl halides, halogen-substituted alkanes are named systematically as haloalkanes, treating the halogen as a substituent on a parent alkane chain. There are three steps:
- Find the longest chain, and name it as the parent. If a double or triple bond is present, the parent chain must contain it.
- Number the carbons of the parent chain beginning at the end nearer the first substituent, whether alkyl or halo. Assign each substituent a number according to its position on the chain. Figure 20.5f. provides two examples on how to number the carbon chain with either an alkyl or halo first appearing in the chain.
Figure 20.5f. Numbering the carbon chain using 5-Bromo-2,4-dimethylheptane and 2-Bromo-4,5-deimethylheptane as examples (credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0). - If different halogens are present, number each one and list them in alphabetical order when writing the name as shown in Figure 20.5g.
Figure 20.5g. Numbering the carbon chain 1-Bromo-3-chloro-4-methylpentane (credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0). - If the parent chain can be properly numbered from either end by step 2, begin at the end nearer the substituent that has alphabetical precedence. Figure 20.5h. shows an example of alphabetical precedence.
Figure 20.5h. An example of alphabetical precedence of 2-Bromo-5-methylhexane (credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0). - In addition to their systematic names, many simple alkyl halides are also named by identifying first the alkyl group and then the halogen. For example in Figure 20.5i., CH3I can be called either iodomethane or methyl iodide. Such names are well entrenched in the chemical literature and in daily usage, but they won’t be used in this book.
In summary, the common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide. The IUPAC system uses the name of the parent alkane with a prefix indicating the halogen substituents, preceded by number indicating the substituent’s location. The prefixes are fluoro-, chloro-, bromo-, and iodo-. Thus CH3CH2Cl has the common name ethyl chloride and the IUPAC name chloroethane. Alkyl halides with simple alkyl groups (one to four carbon atoms) are often called by common names. Those with a larger number of carbon atoms are usually given IUPAC names.
Example 20.5a
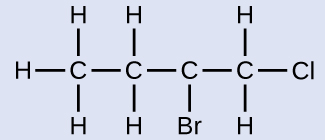
Name the molecule whose structure is shown here:
Solution
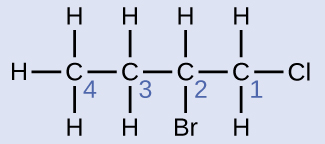
The four-carbon chain is numbered from the end with the chlorine atom. This puts the substituents on positions 1 and 2 (numbering from the other end would put the substituents on positions 3 and 4). Four carbon atoms means that the base name of this compound will be butane. The bromine at position 2 will be described by adding 2-bromo-; this will come at the beginning of the name, since bromo- comes before chloro- alphabetically. The chlorine at position 1 will be described by adding 1-chloro-, resulting in the name of the molecule being 2-bromo-1-chlorobutane.
Example & image source: General Chemistry 1 & 2 , CC BY 4.0.
Example 20.5b
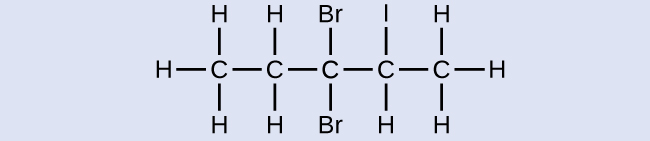
Name the following molecule:
Solution
3,3-dibromo-2-iodopentane
Example & image source: General Chemistry 1 & 2 , CC BY 4.0.
Give the common and IUPAC names for each compound.
- CH3CH2CH2Br
- (CH3)2CHCl
Solution
- The alkyl group (CH3CH2CH2–) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon atom to which the Br atom is attached, so the IUPAC name is 1-bromopropane.
- The alkyl group [(CH3)2CH–] has three carbon atoms, with a chlorine (Cl) atom attached to the middle carbon atom. The alkyl group is therefore isopropyl, and the common name of the compound is isopropyl chloride. For the IUPAC name, the Cl atom (prefix chloro-) attached to the middle (second) carbon atom of a propane chain results in 2-chloropropane.
Exercise 20.5a
Give common and IUPAC names for each compound.
- CH3CH2I
- CH3CH2CH2CH2F
Check Your Answer [1]
Give the IUPAC name for each compound.
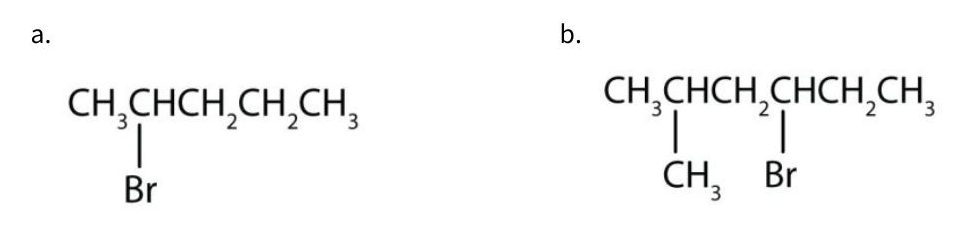
Solution
- The parent alkane has five carbon atoms in the longest continuous chain; it is pentane. A bromo (Br) group is attached to the second carbon atom of the chain. The IUPAC name is 2-bromopentane.
- The parent alkane is hexane. Methyl (CH3) and bromo (Br) groups are attached to the second and fourth carbon atoms, respectively. Listing the substituents in alphabetical order gives the name 4-bromo-2-methylhexane.
Exercise 20.5b
Give the IUPAC name for each compound.
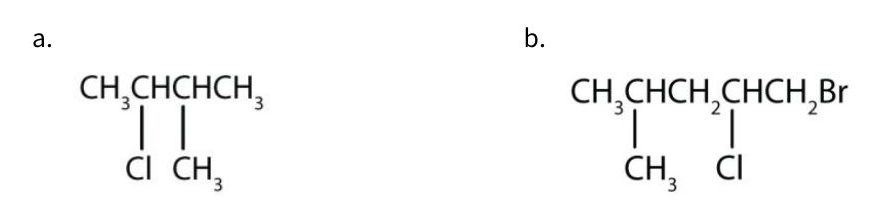
Check Your Answer[2]
For an overview on how to name alkyl halides, watch the video Naming Alkyl Halides – IUPAC Nomenclature below.
Watch Naming Alkyl Halides – IUPAC Nomenclature – YouTube (12 min)
Video source: The Organic Chemistry Tutor. (2018, April 21). Naming Alkyl Halides – IUPAC Nomenclature – YouTube [Video]. YouTube.
A wide variety of interesting and often useful compounds have one or more halogen atoms per molecule. For example, methane (CH4) can react with chlorine (Cl2), replacing one, two, three, or all four hydrogen atoms with Cl atoms. Several halogenated products derived from methane and ethane (CH3CH3) are listed in Table 20.5a., along with some of their uses.
| Formula | Derived from | Common Name | IUPAC Name | Some Important Uses |
|---|---|---|---|---|
| CH3Cl | CH4 | methyl chloride | chloromethane | refrigerant; the manufacture of silicones, methyl cellulose, and synthetic rubber |
| CH2Cl2 | CH4 | methylene chloride | dichloromethane | laboratory and industrial solvent |
| CHCl3 | CH4 | chloroform | trichloromethane | industrial solvent |
| CCl4 | CH4 | carbon tetrachloride | tetrachloromethane | dry-cleaning solvent and fire extinguishers (but no longer recommended for use) |
| CBrF3 | CH4 | halon-1301 | bromotrifluoromethane | fire extinguisher systems |
| CCl3F | CH4 | chlorofluorocarbon-11 (CFC-11) | trichlorofluoromethane | foaming plastics |
| CCl2F2 | CH4 | chlorofluorocarbon-12 (CFC-12) | dichlorodifluoromethane | refrigerant |
| CH3CH2Cl | CH3CH3 | ethyl chloride | chloroethane | local anesthetic |
| ClCH2CH2Cl | CH3CH3 | ethylene dichloride | 1,2-dichloroethane | solvent for rubber |
| CCl3CH3 | CH3CH3 | methylchloroform | 1,1,1-trichloroethane | solvent for cleaning computer chips and molds for shaping plastics |
Spotlight on Everyday Chemistry: Halogenated Hydrocarbons Risks and Benefits
Once widely used in consumer products, many chlorinated hydrocarbons are suspected carcinogens (cancer-causing substances) and also are known to cause severe liver damage. An example is carbon tetrachloride (CCl4), once used as a dry-cleaning solvent and in fire extinguishers but no longer recommended for either use. Even in small amounts, its vapor can cause serious illness if exposure is prolonged. Moreover, it reacts with water at high temperatures to form deadly phosgene (COCl2) gas, which makes the use of CCl4 in fire extinguishers particularly dangerous.
Ethyl chloride, in contrast, is used as an external local anesthetic. When sprayed on the skin, it evaporates quickly, cooling the area enough to make it insensitive to pain. It can also be used as an emergency general anesthetic.
Bromine-containing compounds are widely used in fire extinguishers and as fire retardants on clothing and other materials. Because they too are toxic and have adverse effects on the environment, scientists are engaged in designing safer substitutes for them, as for many other halogenated compounds.
Halomon (IUPAC name (3S,6R)-6-Bromo-3-(bromomethyl)-2,3,7-trichloro-7-methyloct-1-ene) is a pentahalogenated alkene. Halomon as shown in Figure 20.5j., has been isolated from the red alga Portieria hornemannii and found to have anticancer activity against several human tumor cell lines.
Links to Enhanced Learning
For more examples, visit Haloalkanes and Names and Properties of Alkyl Halides by LibreTextsChemistry.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from:
- “18.1 Hydrocarbons” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “12.8: Halogenated Hydrocarbons” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- “10.1 Names and Structures of Alkyl Halides” and “Chemistry Matters—Naturally Occurring Organohalides” In Organic Chemistry (OpenStax) by John McMurray, CC BY-NC-SA 4.0. Access for free at Organic Chemistry (OpenStax)
References cited in-text
Anderson, C. C., & Rayner-Canham, G. (2022, Fall). PFOS: The newest Arctic pollutant. Chem 13 News Magazine.

