26.4 Basicity of Amines
Learning Objectives
By the end of this section, you will be able to:
- Name the typical reactions that take place with amines.
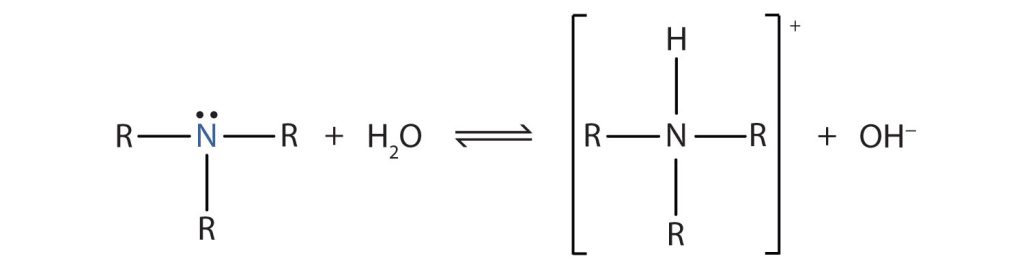
Like ammonia (NH3), amines are weak bases due to the lone pair of electrons on their nitrogen atoms that can accept a proton from water to form substituted ammonium (NH4+) ions and hydroxide (OH−) ions (Figure 26.4a.).
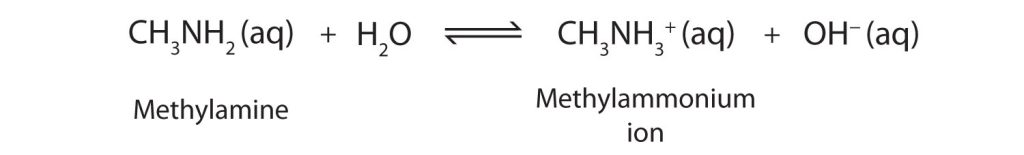
As a specific example, methylamine reacts with water to form the methylammonium ion and the OH− ion (Figure 26.4b.).
The basicity of an amine’s nitrogen atom plays an important role in much of the compound’s chemistry. Amine functional groups are found in a wide variety of compounds, including natural and synthetic dyes, polymers, vitamins, and medications such as penicillin and codeine. They are also found in many molecules essential to life, such as amino acids, hormones, neurotransmitters, and DNA. For more information on DNA and it’s structure see Chapter 28.4 Nucleic Acids and DNA.
Nearly all amines, including those that are not very soluble in water, will react with strong acids to form salts soluble in water (Figure 26.4c.).
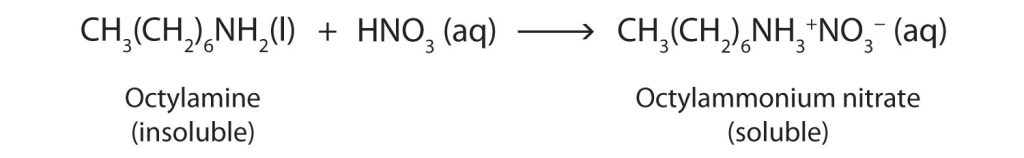
Amine salts are named like other salts: the name of the cation is followed by the name of the anion.
Salts of aniline are properly named as anilinium compounds, but an older system, still in use for naming drugs, identifies the salt of aniline and hydrochloric acid as “aniline hydrochloride.” These compounds are ionic—they are salts—and the properties of the compounds (solubility, for example) are those characteristics of salts. Many drugs that are amines are converted to hydrochloride salts to increase their solubility in aqueous solution.
Example 26.4a
What are the formulas of the acid and base that react to form [CH3NH2CH2CH3]+CH3COO−?
Solution
The cation has two groups—methyl and ethyl—attached to the nitrogen atom. It comes from ethylmethylamine (CH3NHCH2CH3). The anion is the acetate ion. It comes from acetic acid (CH3COOH).
Exercise 26.4a
What are the formulas of the acid and base that react to form (CH3CH2CH2)3NH+I−?
Check Your Answer:[1]
Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey from:
- “20.4: Amines and Amides” In Chemistry 2e (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson is licensed under CC BY 4.0. Access for free at Chemistry 2e (OpenStax) .
- “16.5: Basicity of Amines” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0
- tripropylamine and hydroiodic acid. Such reactions are common for stabilizing amine in medicine, cocaine and opioids. The nitrogen in this molecule becomes the central atom surrounded by three propyl carbon chains and the H+I- ions making the molecule net neutral overall. ↵

