27.4 Addition Polymerization
Learning Objectives
By the end of this section, you will be able to:
- Define addition polymerization.
- Draw the structure of a polymer from its monomer.
- Know the uses/applications of common addition polymers.
Addition polymerization is one method of forming polymers. In addition polymerization, the monomer molecules bond to each other without the loss of any other atoms. Addition polymers from alkene monomers or substituted alkene monomers are the biggest groups of polymers in this class. In some cases, addition polymerization can open a ring-based monomer without the loss of any small molecules.
Process of Addition Polymerization
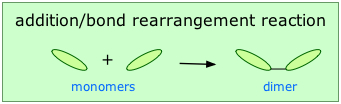
Addition or chain-growth polymerization involves the rearrangement of bonds within the monomer in such a way that the monomers link up directly with each other. This is represented in Figure 27.4a. where two monomers are covalently bonded together to form a dimer (two monomer units joined).
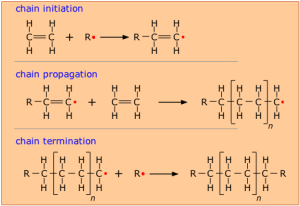
In order to make this happen, an initiator (chemically active molecule) is needed to start what is known as a chain reaction. The manufacture of polyethylene is a very common example of such a process. It employs a free-radical initiator that donates its unpaired electron to the monomer, making the latter highly reactive and able to form a bond with another monomer at this site.
In theory, only a single chain-initiation process needs to take place, and the chain-propagation step then repeats itself indefinitely, but in practice multiple initiation steps are required, and eventually two radicals react to bring the polymerization to a halt. This is called chain termination. See the free radical chain mechanism in Figure 27.4b. As with all polymerizations, chains having a range of molecular weights are produced, and this range can be altered by controlling the pressure and temperature of the process.
Polyethylene Derivatives
Most common plastics and polymers are derived from modified ethylene monomers (Hein et al, 2014). Some common addition polymers are shown in Figure 27.4c. and Table 27.4a. Note that all the monomers have carbon-to-carbon double bonds. Many polymers are mundane (e.g., plastic bags, food wrap, toys, and tableware), but there are also polymers that conduct electricity, have amazing adhesive properties, or are stronger than steel but much lighter in weight.
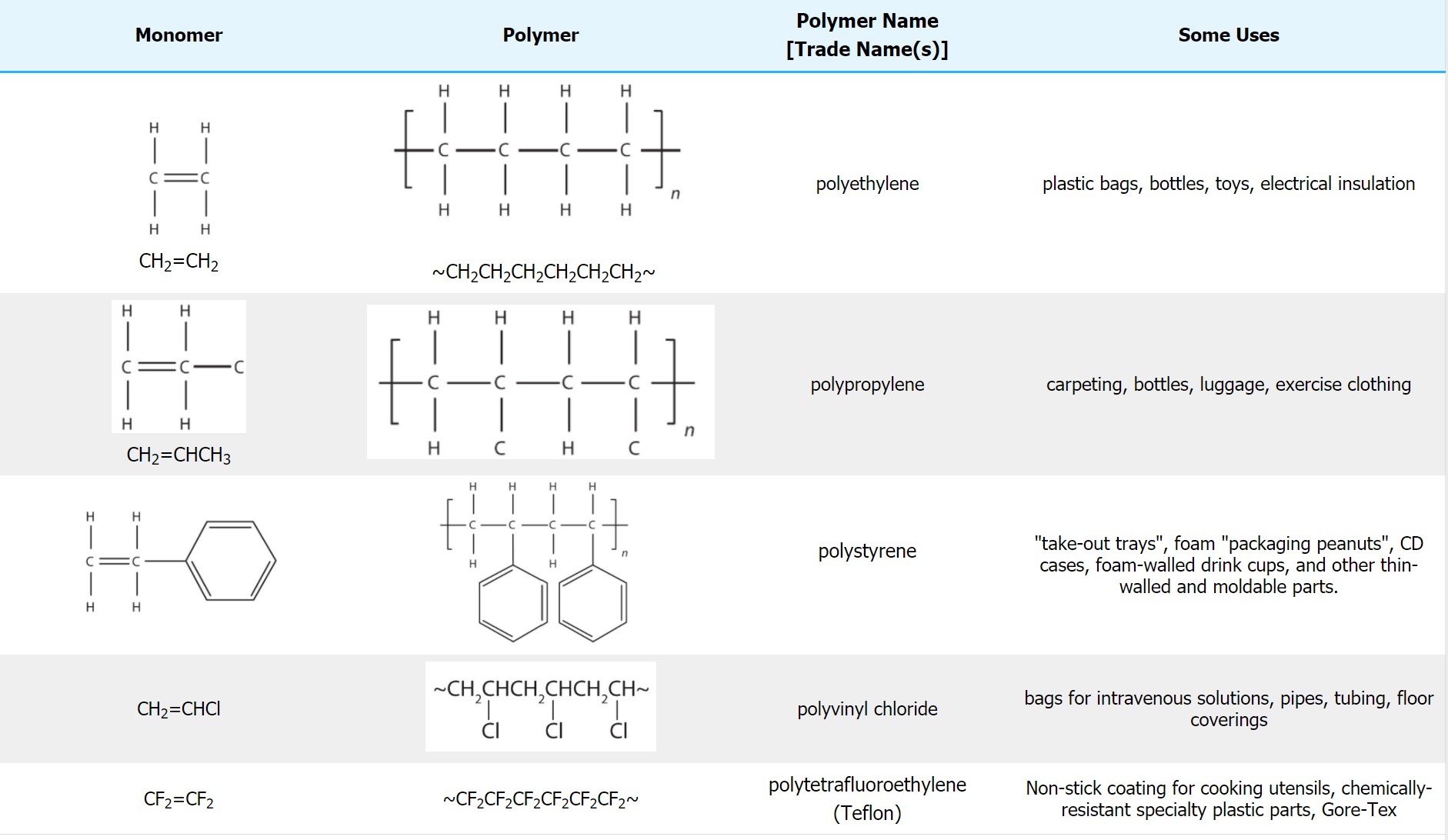
| Monomer | Polymer Name | Trade Name(s) | Uses |
|---|---|---|---|
| H2C=CCl2 | polyvinylidene dichloride | Saran | Clinging food wrap |
| H2C=CH(CN) | polyacrylonitrile | Orlon, Acrilan, Creslan | Fibers for textiles, carpets, upholstery |
| H2C=CH(OCOCH3) | polyvinyl acetate | Elmer’s glue – Silly Putty Demo | |
| H2C=CH(OH) | polyvinyl alcohol | Ghostbusters Demo | |
| H2C=C(CH3)COOCH3 | polymethyl methacrylate | Plexiglass, Lucite | Stiff, clear, plastic sheets, blocks, tubing, and other shapes |
| H2C=CH-C(CH3)=CH2 | polyisoprene | natural or some synthetic rubber | applications similar to natural rubber |
| H2C=CH-CH=CH2 | polybutadiene | polybutadiene synthetic rubber | select synthetic rubber applications |
| H2C=CH-CCl=CH2 | polychloroprene | Neoprene | chemically-resistant rubber |
Table Source: “10.3: Addition Polymerization – One + One + One + … Gives One!” In Map: Chemistry for Changing Times (Hill and McCreary), CC BY-NC-SA 4.0
Polypropylene
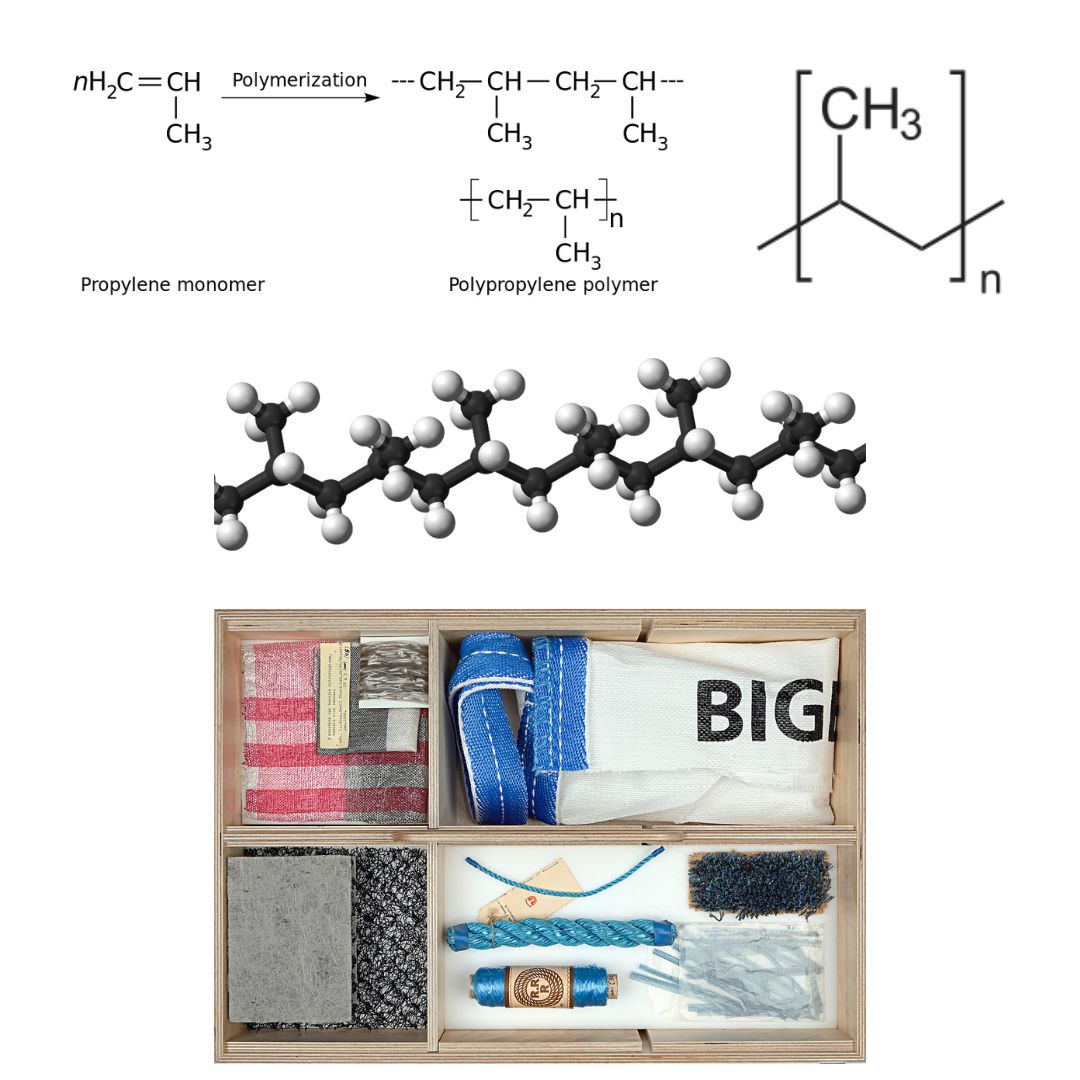
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene. Phillips Petroleum chemists J. Paul Hogan and Robert Banks first polymerized propylene in 1951. The orientation of the third carbon in the propylene monomer results in different version of polypropylene that vary in properties.
Propylene was first polymerized to a crystalline isotactic polymer by Giulio Natta as well as by the German chemist Karl Rehn in March 1954. Polypropylene is used alone or as a copolymer, usually with ethylene. These polymers have an exceptionally wide range of uses — rope, binder covers, plastic bottles, staple yarns, non-woven fabrics, electric kettles. When uncoloured, it is translucent but not transparent. Its resistance to fatigue makes it useful for food containers and their lids, and flip-top lids on bottled products such as ketchup.
After polyethylene, polypropylene is the most profitable plastic with revenues expected to exceed US$145 billion by 2019. The sales of this material are forecast to grow at a rate of 5.8% per year until 2021. Polypropylene is produced by the chain-growth polymerization of propylene. Figure 27.4d. show the polymerization of polypropylene, the line structure of polypropylene, a 3D rendering of polypropylene and examples of materials made from polypropylene.
Polystyrene
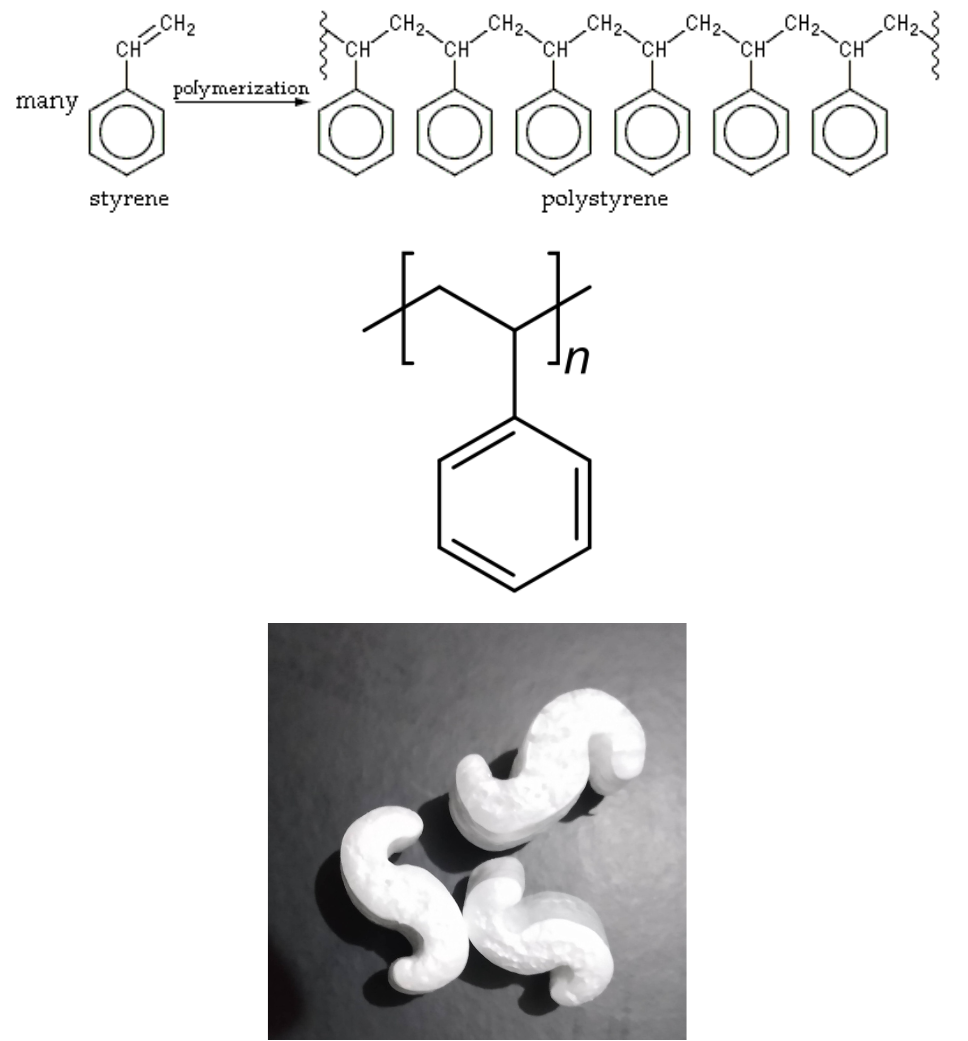
Polystyrene was discovered in 1839 by Eduard Simon, an apothecary from Berlin. In 1941, Dow Chemical invented a Styrofoam process. Polystyrene is transparent but rather brittle, and yellows under UV light. Widely used for inexpensive packaging materials and “take-out trays”, foam “packaging peanuts”, CD cases, foam-walled drink cups, and other thin-walled and moldable parts.
Expanded polystyrene (EPS) is a rigid and tough, closed-cell foam with a normal density range of 11 to 32 kg/m3. It is usually white and made of pre-expanded polystyrene beads. EPS is used for food containers, molded sheets for building insulation, and packing material either as solid blocks formed to accommodate the item being protected or as loose-fill “peanuts” cushioning fragile items inside boxes. EPS is colloquially called “styrofoam” in the United States and Canada, an incorrectly applied genericization of Dow Chemical’s brand of extruded polystyrene.
Polystyrene results when styrene monomers interconnect. In the polymerization, the carbon–carbon π bond of the vinyl group is broken and a new carbon–carbon σ bond is formed, attaching to the carbon of another styrene monomer to the chain. Figure 27.4e. shows the polymerization of polystyrene, the line structure of polystyrene and an example of materials made from polystyrene.
Polyvinyl Chloride
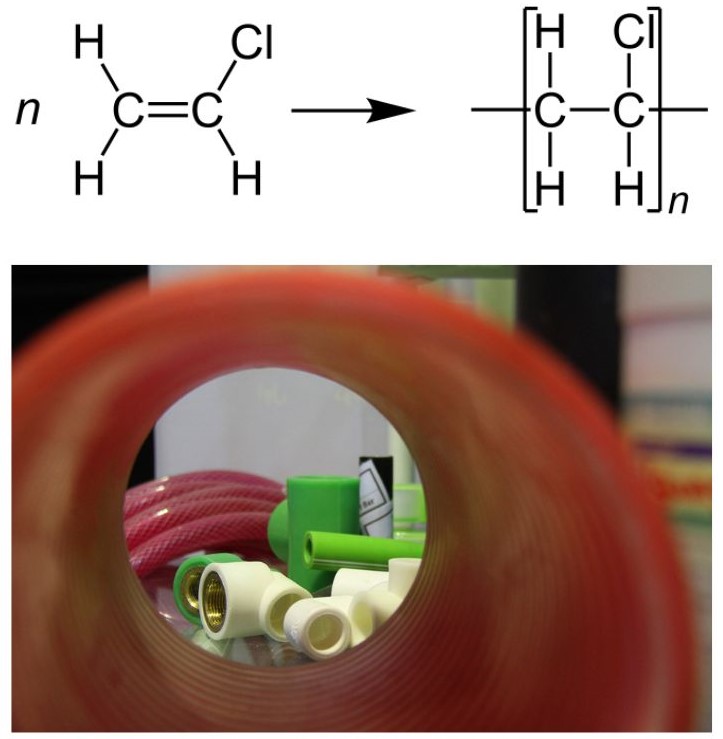
PVC was accidentally synthesized in 1872 by German chemist Eugen Baumann. The polymer appeared as a white solid inside a flask of vinyl chloride that had been left exposed to sunlight. Polyvinyl chloride (PVC) is the world’s third-most widely produced synthetic plastic polymer, after polyethylene and polypropylene. About 40 million tonnes are produced per year. Polyvinyl chloride is one of the world’s most widely used polymers. By itself it is quite rigid and used in construction materials such as pipes, house siding, flooring. Addition of plasticizers make it soft and flexible for use in upholstery, electrical insulation, shower curtains and waterproof fabrics. There is some effort being made to phase out this polymer owing to environmental concerns. Figure 27.4f. shows the polymerization of vinyl chloride to form PVC as well as examples of PVC piping.
Polytetrafluorethylene (PTFE): The Nonstick Coating
Spotlight on Everyday Chemistry: Teflon
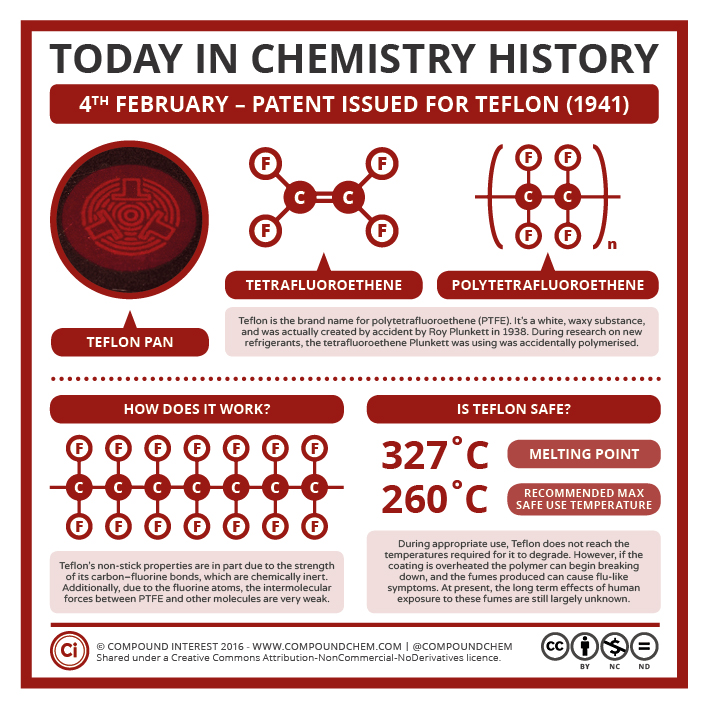
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. The best-known brand name of PTFE-based formulas is Teflon (Infographic 27.4a) Chemours, a spin-off of DuPont, originally discovered the compound in 1938. This highly crystalline fluorocarbon is exceptionally inert to chemicals and solvents. Water and oils do not wet it, which accounts for its use in cooking ware and other anti-stick applications, including personal care products.
These properties — non-adhesion to other materials, non-wetability, and very low coefficient of friction (“slipperyness”) — have their origin in the highly electronegative nature of fluorine whose atoms partly shield the carbon chain. Fluorine’s outer electrons are so strongly attracted to its nucleus that they are less available to participate in London (dispersion force) interactions.
Example 27.4a
Draw the polymer that results from the polymerization of tetrafluoroethylene.
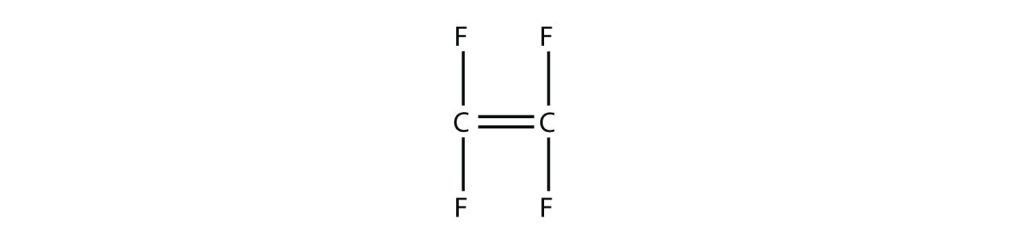
Answer:
In the case of this monomer, the double bond opens up and joins to other monomers, just as with ethylene. The polymer that is made has this structure:
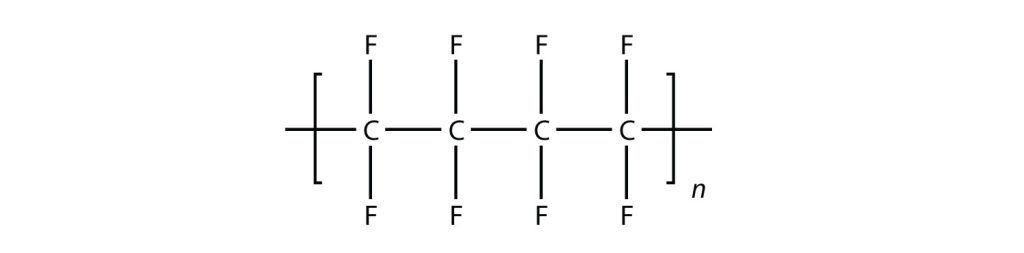
Exercise & Image credits: Introductory Chemistry (V 1.0), CC BY-NC-SA 3.0
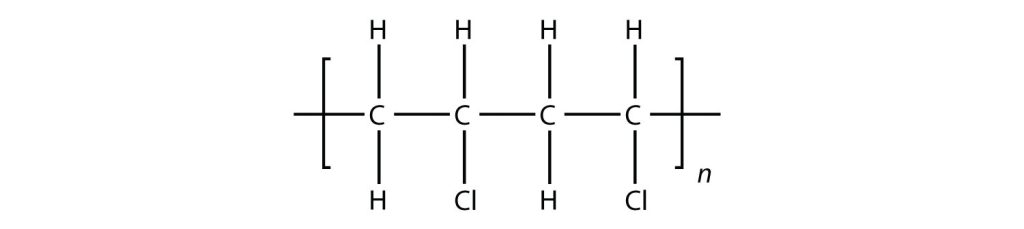
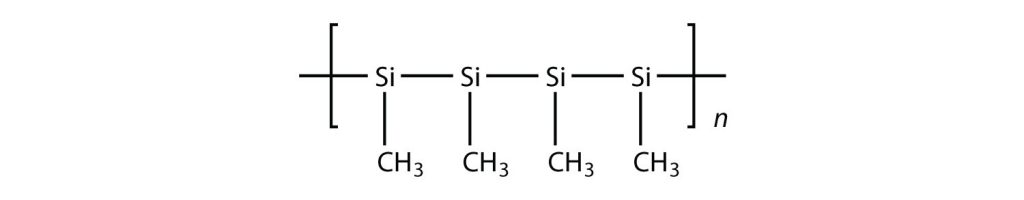
Exercise 27.4a
Draw the polymer that results from the polymerization of the following monomers. (Assume Si behaves the same as C)
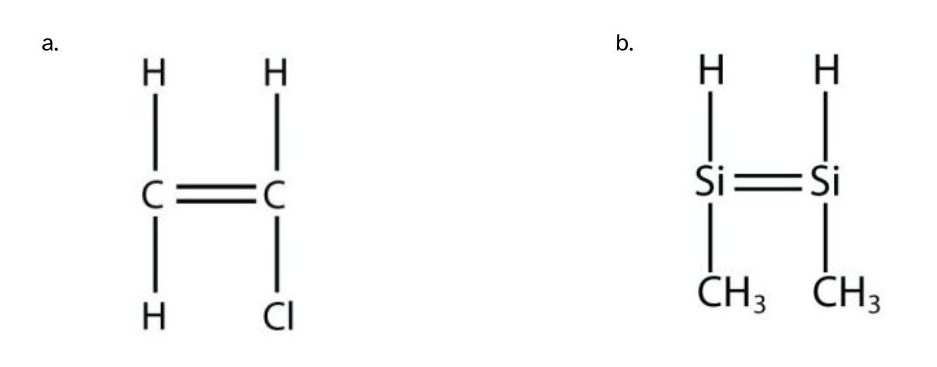
Exercise & Image (including solution images) credits: Introductory Chemistry (V 1.0), CC BY-NC-SA 3.0
Check Your Answers:[1]
Exercise 27.4b
In this image, identify which polymers are addition polymers. How are the other polymers formed?
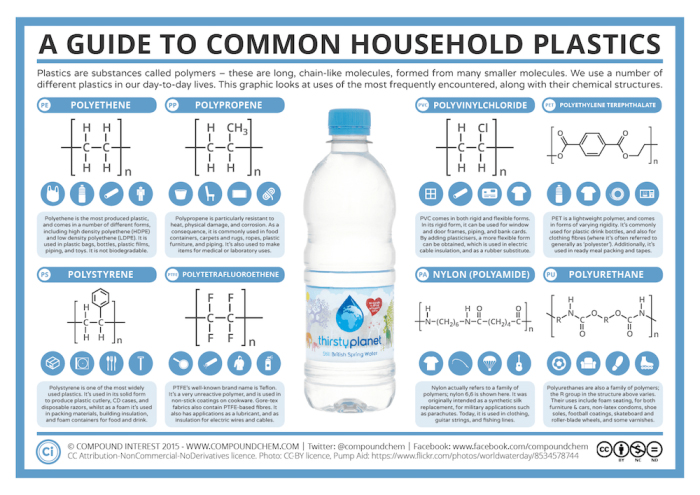
Check Your Answers: [2]
Attribution & References
Except where otherwise noted, this page has been adapted by Samantha Sullivan Sauer from
- “10.3: Addition Polymerization – One + One + One + … Gives One!” In Map: Chemistry for Changing Times (Hill and McCreary) by LibreTexts, licensed under CC BY-NC-SA 4.0. Attributions from original text:
-
- Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook
- Beginning Chemistry (Ball), CC BY-NC-SA 3.0, a LibreTexts version of Beginning Chemistry (v 1.0).
- Basics of General, Organic, and Biological Chemistry (Ball et al.)by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- Marisa Alviar-Agnew (Sacramento City College)
- Charles Ophardt (Professor Emeritus, Elmhurst College); Virtual Chembook
- Wikipedia
- “16.6 Polymers” In Introductory Chemistry by Saylor Academy, CC BY-NC-SA 3.0
-
References cited in-text
Hein, M., Pattison, S., Arena, S., & Best, L. R. (2014). Introduction to General, Organic, and Biochemistry (11th ed.). Wiley.
chemically active molecule
process of reactions repeating themselves following initiation
a neutral chemical species that contains an odd number of electrons and thus has a single, unpaired electron in one of its orbitals
two radicals react to bring the polymerization to a halt
also called polypropene; made from the monomer propylene (propene)
two or more different monomers that form a polymer in a specific ratio
polymer produced from vinyl chloride monomer
synthetic fluoropolymer of tetrafluoroethylene