26.1 Amines – Structure and Naming
Learning Objectives
By the end of this section, you will be able to:
- Identify the general structure for an amine.
- Identify the functional group for amines.
- Determine the structural feature that classifies amines as primary, secondary, or tertiary.
- Use nomenclature systems to name amines.
Amines are molecules that contain carbon-nitrogen bonds. The nitrogen atom in an amine has a lone pair of electrons and three bonds to other atoms, either carbon or hydrogen. Various nomenclatures are used to derive names for amines, but all involve the class-identifying suffix –ine as illustrated here for a few simple examples:
In some amines, the nitrogen atom replaces a carbon atom in an aromatic hydrocarbon. Pyridine (Figure 26.1b.) is one such heterocyclic amine. A heterocyclic compound contains atoms of two or more different elements in its ring structure.
Classifying Amines
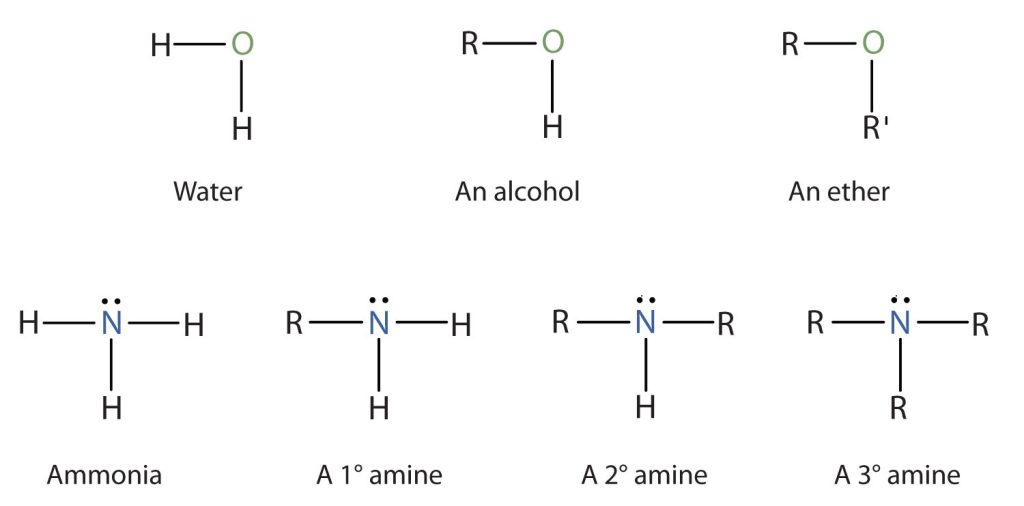
To classify alcohols, we look at the number of carbon atoms bonded to the carbon atom bearing the OH group, not the oxygen atom itself. Thus, although isopropylamine looks similar to isopropyl alcohol, the former is a primary amine, while the latter is a secondary alcohol (Figure 26.1e.).

Compounds containing a nitrogen atom with four attached groups also exist, but the nitrogen atom must carry a formal positive charge. Such compounds are called quaternary ammonium salts (Figure 26.1f.).
Naming Amines
Primary amines are named in two main ways using the IUPAC system. They can either be named as alkylamines or as alkanamines. Most 1o amines which are attached to linear alkanes, cycloalkanes, and alkyl groups with common names tend to be named as alkylamines.
Steps:
- Identify the longest carbon chain bonded to the amine nitogen.
- The alkyl group is named as a substituent (prefix + alkyl).
- The suffix amine is added to the end.
Primary also have several common names. You might recall that the aromatic phenylamine, H2N–C6H5, has the common name aniline (Figure 26.1g.).
Alternatively, primary amines tend to be named as alkanamines (Figure 26.1h.).
Steps:
- Identify the longest carbon chain bonded to the amine nitrogen.
- Identify the substituents.
- Number the parent chain, giving the amine the lowest number.
- Put all details together and ensure the substituents are in alphabetical order.
Amines with more than one functional group are named by considering the −NH2 as an amino substituent on the parent molecule (Figure 26.1i.).
Symmetrical secondary and tertiary amines are named by adding the prefix di– or tri– to the alkyl group (Figure 26.1j.).
Unsymmetrically substituted secondary and tertiary amines are referred to as N-substituted primary amines. The largest alkyl group takes the parent name, and the other alkyl groups are considered N-substituents on the parent (N because they’re attached to nitrogen) (Figure 26.1k.).
The common names for simple aliphatic amines consist of an alphabetic list of alkyl groups attached to the nitrogen atom, followed by the suffix –amine. (Systematic names are often used by some chemists.) The amino group (NH2) is named as a substituent in more complicated amines, such as those that incorporate other functional groups or in which the alkyl groups cannot be simply named.
Example 26.1a
Name and classify each compound.
- CH3CH2CH2NH2
-
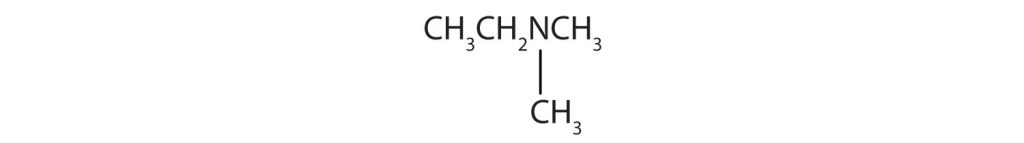
(Credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0.) - CH3CH2CH2NHCH3
- CH3CH2NHCH2CH
Solution
- There is only one alkyl group attached to the nitrogen atom, so the amine is primary. A group of three carbon atoms (a propyl group) is attached to the NH2 group through an end carbon atom, so the name is propylamine.
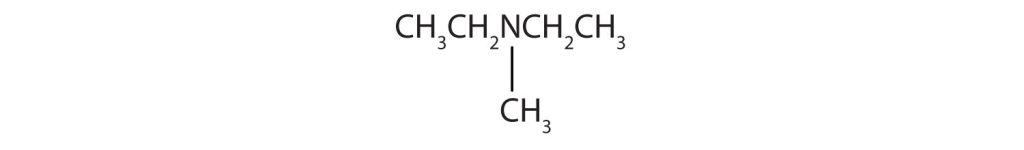
- There are two methyl groups and one ethyl group on the nitrogen atom. The compound is ethyldimethylamine, a tertiary amine.
- There are two ethyl groups attached to the nitrogen atom; the amine is secondary, so the compound is diethylamine.
- The nitrogen atom has a methyl group and a propyl group, so the compound is methylpropylamine, a secondary amine.
Exercise 26.1a
Draw the structure for each compound and classify.
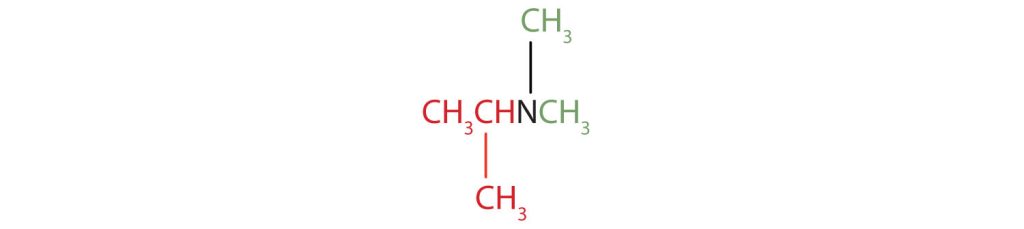
- isopropyldimethylamine
- dipropylamine
Check Your Answers:[1]
Exercise & solution image source: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0.
Exercise 26.1b
Exercise 26.1c
Name and classify each compound.
- CH3CH2CH2CH2NH2
- CH3CH2CH2NHCH2CH2 CH3
Check Your Answers:[3]
Exercise and image credits: Introduction to Chemistry: GOB (V. 1.0)., CC BY-NC-SA 3.0
Example 26.1b
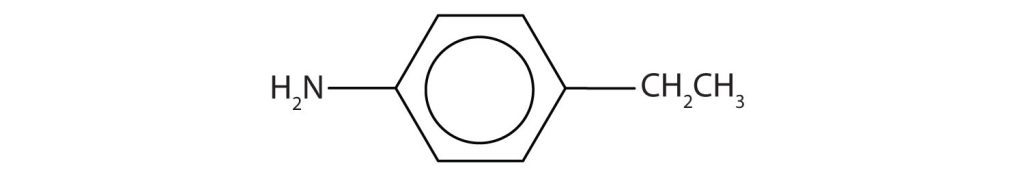
Draw the structure for p-ethylaniline and classify.
Solution
The compound is a derivative of aniline. It is a primary amine having an ethyl group located para to the amino (NH2) group.
Exercise 26.1d
Draw the structure for 2-amino-3-methylpentane.
Check Your Answer:[4]
Exercise & solution image source: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0.
Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey from:
- 20.4 Amines and Amides In Chemistry 2e (OpenStax) by Flowers, Paul., Theopold, Klaus., Langley, Richard., R. Robinson, William R., licensed under CC BY 4.0. Access for free at Chemistry 2e (Open Stax)
- “16.1: Classifying Amines” & “16.2: Naming and Drawing Amines” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0
- “24.1 Naming Amines” In Organic Chemistry (OpenStax) by John McMurry, CC BY-NC-SA 4.0. Access for free at Organic Chemistry (OpenStax)
-
- The name indicates that there are an isopropyl group (in red) and two methyl groups (in green) attached to the nitrogen atom; the amine is tertiary.
- The name indicates that there are two propyl groups attached to the nitrogen atom; the amine is secondary. (The third bond on the nitrogen atom goes to a hydrogen atom.) CH3CH2CH2NHCH2CH2CH3
↵
- The name indicates that there are an isopropyl group (in red) and two methyl groups (in green) attached to the nitrogen atom; the amine is tertiary.
-
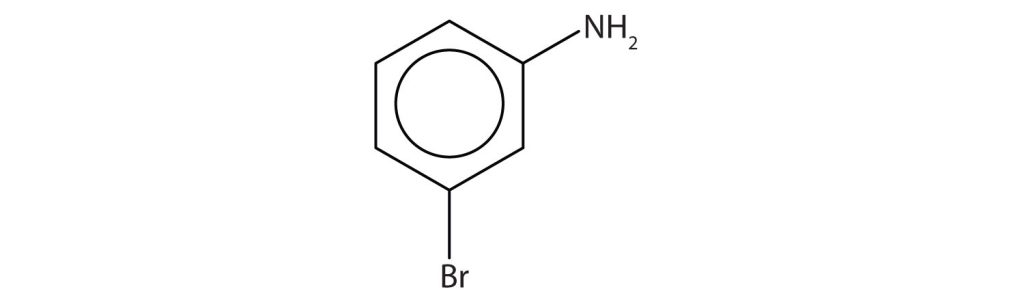
The benzene ring with an amino (NH2) group is aniline. The compound is named as a derivative of aniline: 3-bromoaniline or m-bromoaniline.
↵
-
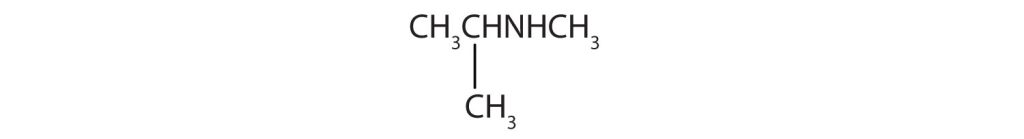
1) secondary amine, N-methylisopropylamine or methylisopropylamine 2) tertiary amine, N-ethyl-N-methylethylamine or diethylmethylamine 3) primary amine, butylamine, 4) secondary amine, n-propylpropylamine or dipropylamine
↵
-
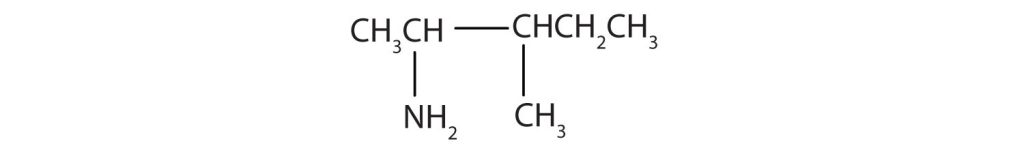
Always start with the parent compound: draw the pentane chain. Then attach a methyl group at the third carbon atom and an amino group at the second carbon atom. ↵
an amine containing one alkyl group on the central nitrogen atom.
has two alkyl groups on the central nitrogen.
has three alkyl groups on the central nitrogen atom.