27.9 Plastics and the Environment
Learning Objectives
By the end of this section, you will be able to:
- Describe the problems associated with plastics.
- Identify potential improvements in the formation and end-life of plastics.
Watch What really happens to the plastic you throw away – Emma Bryce on YouTube (4 mins)
Video source: TED-ED. (2015, April 21). What really happens to the plastic you throw away – Emma Bryce [Video]. YouTube.
Problems with Plastics
Due to their low cost, ease of manufacture, versatility, and imperviousness to water, plastics are used in a multitude of products of different scale, including paper clips and spacecraft. They have prevailed over traditional materials such as wood, stone, horn and bone, leather, metal, glass, and ceramic. However, there are numerous problems encountered with plastic use.
Small-molecule release
Many kinds of polymers contain small molecules — either unreacted monomers, or substances specifically added (plasticizers, UV absorbers, flame retardants, etc.) to modify their properties. Many of these smaller molecules are able to diffuse through the material and be released into any liquid or air in contact with the plastic and eventually into the aquatic environment. Those that are used for building materials (in mobile homes, for example) can build up in closed environments and contribute to indoor air pollution.
Residual monomer
Formation of long polymer chains is a complicated and somewhat random process that is never perfectly stoichiometric. It is therefore not uncommon for some unreacted monomer to remain in the finished product. Some of these monomers, such as formaldehyde, styrene (from polystyrene, including polystyrene foam food take-out containers), vinyl chloride, and bisphenol-A (from polycarbonates) are known carcinogens. Although there is little evidence that the small quantities that diffuse into the air or leach out into fluids pose a quantifiable health risk, people are understandably reluctant to tolerate these exposures, and public policy is gradually beginning to regulate them.
For example, perfluorooctanoic acid (PFOA), the monomer from which Teflon is made, has been the subject of a 2004 lawsuit against a DuPont factory that contaminated groundwater. Small amounts of PFOA have been detected in gaseous emissions from hot fluorocarbon products.
Decomposition products
Most commonly used polymers are not readily biodegradable, particularly under the anaerobic conditions of most landfills. Also, what decomposition does occur will combine with rainwater to form leachates that can contaminate nearby streams and groundwater supplies. Partial photodecomposition, initiated by exposure to sunlight, is a more likely long-term fate for exposed plastics, resulting in tiny broken-up fragments. Many of these materials are less dense than seawater, and once they enter the oceans through coastal sewage outfalls or from marine vessel wastes, they tend to remain there indefinitely.
Open burning of polymeric materials containing chlorine (polyvinyl chloride, for example) is known to release compounds such as dioxins that persist in the environment. Incineration under the right conditions can effectively eliminate this hazard. Disposed products containing fluorocarbons (Teflon-coated ware, some personal-care, waterproofing and anti-stick materials) break down into perfluorooctane sulfonate which has been shown to damage aquatic animals.
Hazards to animals
There are two general types of hazards that polymers can introduce into the aquatic environment. One of these relates to the release of small molecules that act as hormone disrupters as described above. It is well established that small aquatic animals such as fish are being seriously affected by such substances in many rivers and estuarine systems, but details of the sources and identities of these molecules have not been identified. One confounding factor is the release of sewage water containing human birth-control drugs (which have a feminizing effect on sexual development) into many waterways.
The other hazard relates to pieces of plastic waste that aquatic animals mistake for food or become entangled in (Figure 27.9a.).

These dangers occur throughout the ocean but are greatly accentuated in regions known as gyres. These are regions of the ocean in which a combination of ocean currents drives permanent vortices that tend to collect and concentrate floating materials. The most notorious of these are the Great Pacific Gyres (Figure 27.9b.) that have accumulated astounding quantities of plastic waste.

Plastics and Fire Hazards
The term fire (or flame)-retardant as applied to organic (carbon based) materials is intended to refer to reduced fire hazard, as all will burn under certain circumstances. Fabric flammability is an important textile issue, especially for stage drapery that will be used in a public space such as a school, theatre or special event venue. In the United States, federal regulations require that drapery fabrics used in such spaces be certified as flame or fire-retardant. For draperies and other fabrics used in public places, this is known as the NFPA 701 Test, which follows standards developed by the National Fire Protection Association (NFPA). Although all fabrics will burn, some are naturally more resistant to fire than others. Those that are more flammable can have their fire resistance drastically improved by treatment with fire-retardant chemicals. Inherently flame-retardant fabrics such as polyester are commonly used for flame retardant curtain fabrics.
The deaths in fiery crashes of race car drivers Fireball Roberts at Charlotte, and Eddie Sachs and Dave MacDonald at Indianapolis in 1964 led to the use of flame-resistant fabrics such as Nomex. Nomex and related aramid polymers are related to nylon, but have aromatic backbones, and hence are more rigid and more durable. Nomex is an example of a meta variant of the aramids. Kevlar is a para aramid. Unlike Kevlar, Nomex strands cannot align during filament polymerization and has less strength. However, it has excellent thermal, chemical, and radiation resistance for a polymer material.
A Nomex hood is a common piece of racing and firefighting equipment. It is placed on the head on top of a firefighter’s face mask (Figure 27.9c.). The hood protects the portions of the head not covered by the helmet and face mask from the intense heat of the fire. Wildland firefighters wear Nomex shirts and trousers as part of their personal protective equipment during wildfire suppression activities. Racing car drivers wear driving suits constructed of Nomex and or other fire-retardant materials, along with Nomex gloves, long underwear, balaclavas, socks, helmet lining and shoes, to protect them in the event of a fire. Military pilots and aircrew wear flight suits made of over 92 percent Nomex to protect them from the possibility of cockpit fires and other mishaps. Recently, troops riding in ground vehicles have also begun wearing Nomex. Kevlar thread is often used to hold the fabric together at seams. Military tank drivers also typically use Nomex hoods as protection against fire.

Plasticizers and Pollution
Plasticizers or dispersants are additives that increase the plasticity or decrease the viscosity of a material. These substances are compounded into certain types of plastics to render them more flexible by lowering the glass transition temperature. They accomplish this by taking up space between the polymer chains and acting as lubricants to enable the chains to more readily slip over each other (Figure 27.9d.). Many are small enough to be diffusible and a potential source of health problems.
Polyvinyl chloride polymers are one of the most widely plasticized types, and the odours often associated with flexible vinyl materials such as garden hoses, waterbeds, cheap shower curtains, raincoats and upholstery are testament to their ability to migrate into the environment.  The well-known “new car smell” is largely due to plasticizer release from upholstery and internal trim. According to 2014 data, the total global market for plasticizers was 8.4 million metric tonnes including 1.3 million metric tonnes in Europe.
The well-known “new car smell” is largely due to plasticizer release from upholstery and internal trim. According to 2014 data, the total global market for plasticizers was 8.4 million metric tonnes including 1.3 million metric tonnes in Europe.
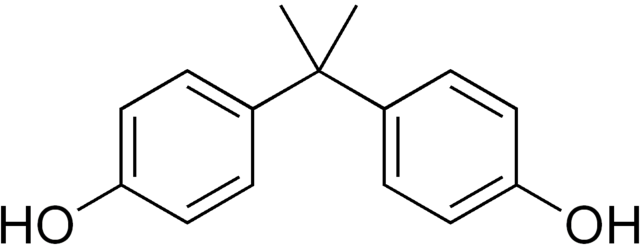
Substantial concerns have been expressed over the safety of some plasticizers, especially because some low molecular weight ortho-phthalates have been classified as potential endocrine disruptors with some developmental toxicity reported. A common plasticizer identified in many common plastic containers was BPA or bisphenol-A (Figure 27.9f.). Due to health concerns, especially with young children, most plastic drinking cups and other kids items are listed as BPA-Free. Heating plastics containing plasticizers can encourage leaching of the chemicals. (Hein et al., 2014)
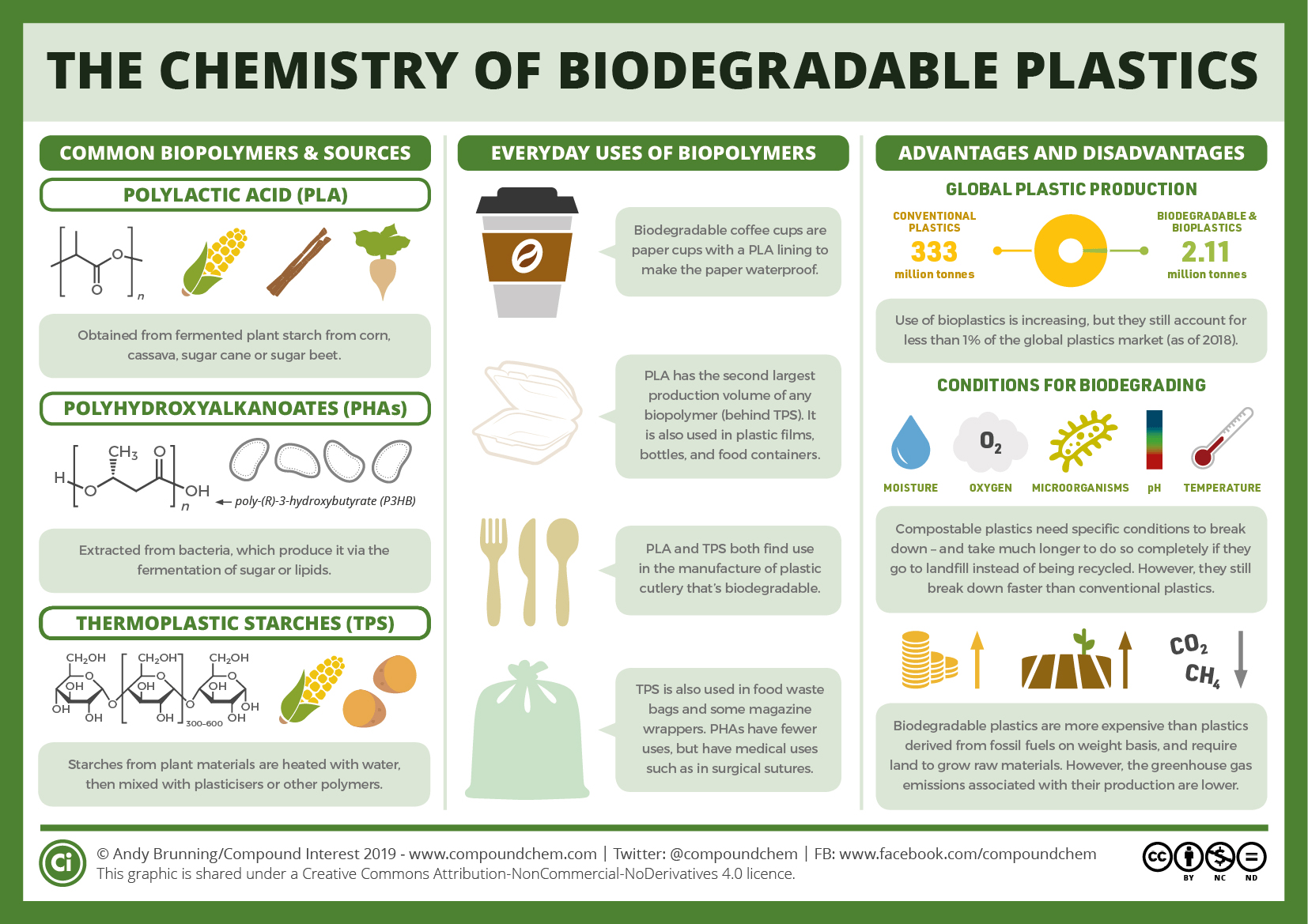
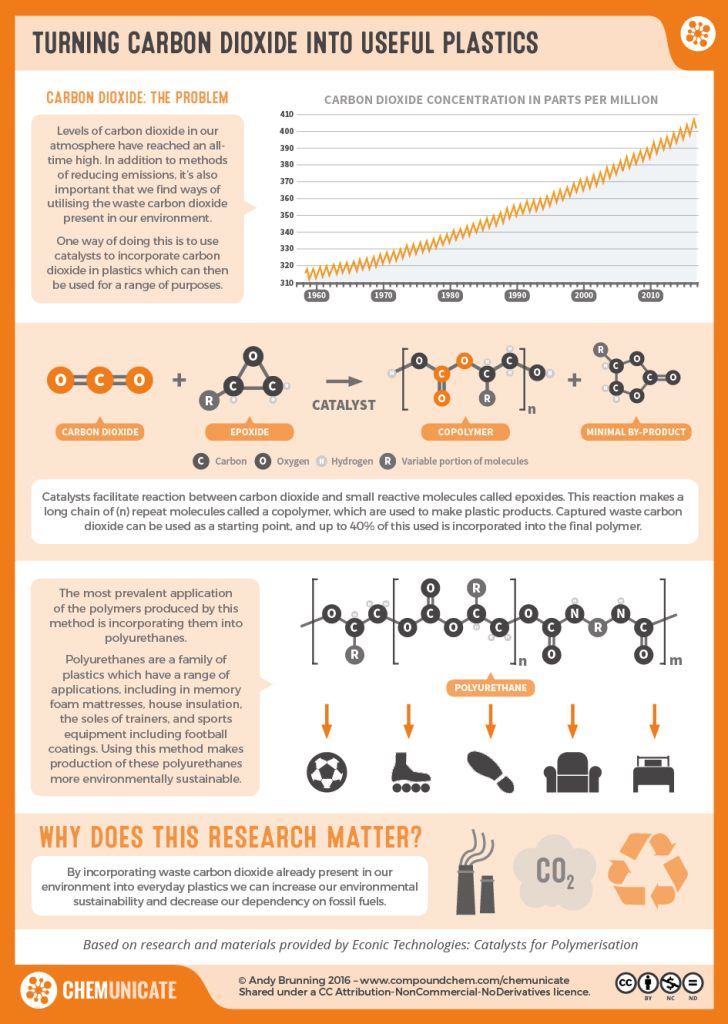
Plastics of the Future
Current research is looking to the plastics of the future. Biodegradable plastics (Infographic 27.9a.) and different uses of plastic waste are regularly being reported. New sources of starting materials for plastics are being used (Infographic 27.9b.).
Links to Enhanced Learning
To read more about new polymers and upcycling polymers, see these suggested articles:
- New family of polymers can be easily recycled and even upcycled | Research | Chemistry World [New tab]
- Plastic packaging waste upcycled into technical materials | Research | Chemistry World [New tab]
- Genetically engineered microbes convert waste plastic into vanillin | Research | Chemistry World [New tab]
- Polyurethane foams given a new lease of life as high-performance 3D printing inks | Research | Chemistry World [New tab]
Attribution & References
Except where otherwise noted, this page has been adapted by Samantha Sullivan Sauer from
- “10.7: Plastics and the Environment” by Marisa Alviar-Agnew (Sacramento City College) In Map: Chemistry for Changing Times (Hill and McCreary) & LibreTexts, licensed under CC BY-NC-SA 4.0. Attributions from original source:
- Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook
- Marisa Alviar-Agnew (Sacramento City College)
- Wikipedia
References cited in text
Hein, M., Pattison, S., Arena, S., & Best, L. R. (2014). Introduction to General, Organic, and Biochemistry (11th ed.). Wiley.
additives that increase the plasticity or decrease the viscosity of a material

