25.4 Ionization and Neutralization of Carboxylic Acids
Learning Objectives
By the end of this section, you will be able to:
- Name the typical reactions that take place with carboxylic acids.
- Describe how carboxylic acids react with basic compounds.
Ionization of Carboxylic Acids
The acidic nature of carboxylic acids, compared to other organic molecules, is due to the fact that the carboxyl group contains hydrogen which in solution in water can be transferred to the water molecule. The carboxylic acid in aqueous solutions acts as a weak acid and will only partially dissociate. The dissociated ions include the corresponding carboxylate anion and the hydronium cation (H3O+) (Figure 25.4a. and 25.4b.). The carboxylate anions are named by replacing the –ic acid ending from the carboxylic acid with –ate, see examples below (Kennepohl et al, n.d.).
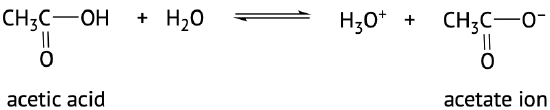
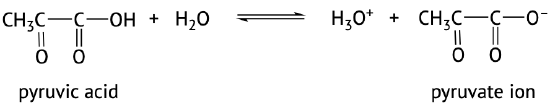
The extent of dissociation of these weak acids in water is described by Ka values. Remember that a compound with a smaller Ka value will be a weaker acid (Figure 25.4c).
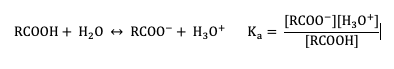
When comparing the acidity of organic and biomolecules, it is useful (and more preferable) to use pKa values instead of Ka values, which are calculated by taking the negative log of Ka: pKa = –log(Ka). When using the pKa scale, it is important to know that weaker acids have larger and more positive pKa values, this is opposite of Ka values. The pKa values of some typical carboxylic acids are listed in Table 25.4a. (Remember that pKa is a log expression, which means that every 1 pKa unit represents a 10-fold change in acidity.)
| Name | Compound | Ka | pKa |
|---|---|---|---|
| formic acid | HCOOH | 1.8 X 10–4 | 3.74 |
| acetic acid | CH3COOH | 1.8 X 10–5 | 4.74 |
| propanoic acid | CH3CH2COOH | 1.3 X 10–5 | 4.89 |
| butanoic acid | CH3CH2CH2COOH | 1.5 X 10–5 | 4.82 |
| chloroacetic acid | ClCH2COOH | 1.4 X 10–3 | 2.85 |
| trichloroacetic acid | Cl3CCOOH | 2.3 X 10–1 | 0.64 |
| hexanoic acid | CH3(CH2)4COOH | 1.3 X 10–5 | 4.89 |
| benzoic acid | C6H5COOH | 6.5 X 10–5 | 4.19 |
| oxalic acid | HOOCCOOH | 5.4 X 10–2 | 1.27 |
| –OOCCOOH | 5.2 X 10–5 | 4.28 | |
| glutaric acid | HOOC(CH2)3COOH | 4.5 X 10–5 | 4.35 |
| –OOC(CH2)3COOH | 3.8 X 10–6 | 5.42 |
Source: Comparison of Carboxylic acids. (Credit: Chem 114: Human Chemistry II (Muíño),CC BY-NC-SA 4.0).
These water-soluble carboxylic acids ionize to form moderately acidic solutions that exhibit the typical properties of acids, such as changing litmus from blue to red (Figure 25.4d). The anion formed when a carboxylic acid dissociates is called the carboxylate anion (RCOO−).
Neutralization of Carboxylic Acids
Carboxylic acids will react with bases such as sodium hydroxide (NaOH), sodium carbonate (Na2CO3), and sodium bicarbonate (NaHCO3) to form water and a carboxylic acid salt:
RCOOH + NaOH(aq) → RCOO−Na+(aq) + H2O
2RCOOH + Na2CO3(aq) → 2RCOO−Na+(aq) + H2O + CO2(g)
RCOOH + NaHCO3(aq) → RCOO−Na+(aq) + H2O + CO2(g)
In these reactions, the carboxylic acids act like inorganic acids: they neutralize basic compounds. With solutions of carbonate (CO32-) and bicarbonate (HCO3–) ions, they also form carbon dioxide gas.
Carboxylic acid salts are named in the same manner as inorganic salts: the name of the cation is followed by the name of the organic anion. The name of the anion is obtained by dropping the –ic ending of the acid name and replacing it with the suffix –ate (Figure 25.4e). This rule applies whether we are using common names or International Union of Pure and Applied Chemistry (IUPAC) names:

The salts of long-chain carboxylic acids are called soaps (Figure 25.4f). We discuss the chemistry of soaps elsewhere.
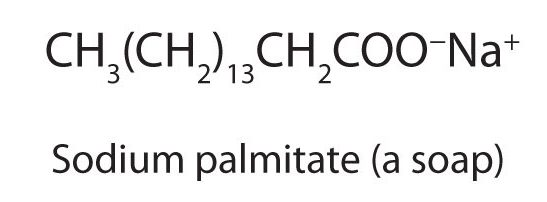
Example 25.4a
Write an equation for each reaction.
- the ionization of propionic acid in water (H2O)
- the neutralization of propionic acid with aqueous sodium hydroxide (NaOH)
Solution:
Propionic acid has three carbon atoms, so its formula is CH2CH2COOH.
- Propionic acid ionizes in water to form a propionate ion and a hydronium (H3O+) ion. CH3CH2COOH(aq) + H2O(ℓ) → CH3CH2COO−(aq) + H3O+(aq)
- Propionic acid reacts with NaOH(aq) to form sodium propionate and water. CH3CH2COOH(aq) + NaOH(aq) → CH3CH2COO−Na+(aq) + H2O(ℓ)
Exercise 25.4a
Write an equation for the reaction of decanoic acid with each compound.
- aqueous sodium hydroxide (NaOH)
- aqueous sodium bicarbonate (NaHCO3)
Check Your Answer[1]
Spotlight on Everyday Chemistry:
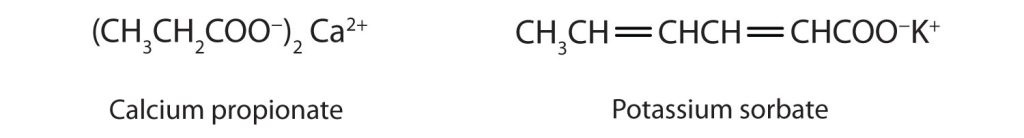
Some organic salts are used as preservatives in food products. They prevent spoilage by inhibiting the growth of bacteria and fungi. Calcium and sodium propionate, for example, are added to processed cheese and bakery goods; sodium benzoate is added to cider, jellies, pickles, and syrups; and sodium sorbate and potassium sorbate are added to fruit juices, sauerkraut, soft drinks, and wine (Figure 25.4g). Look for them on ingredient labels the next time you shop for groceries.
Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey from:
- “15.4: Chemical Properties of Carboxylic Acids- Ionization and Neutralization” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative authored, remixed, and/or curated by Anonymous via source content that was edited to the style and standards of the LibreTexts platform.
- Figures 25.4a & 25.4b are reused from “17.3: Acidity of Carboxylic Acids” In Chem 114: Human Chemistry II (Muíño), shared under an “undeclared” license but assumed to be CC BY-NC-SA 4.0 given the original source material authored, remixed, and/or curated by Anonymous via source content
References cited in-text
Kennepohl, D., Morsch, L., Farmer, S. Reusch, W. (n.d.). 20.2: Structure and properties of carboxylic acids. In Organic Chemistry (Morsch et al.). LibreTexts. CC BY-SA 4.0.
-
- Decanoic acid has 10 carbon atoms. It reacts with NaOH to form a salt and water (H2O). CH3(CH2)8COOH + NaOH(aq) → CH3(CH2)8COO−Na+(aq) + H2O(ℓ)
- With NaHCO3, the products are a salt, H2O, and carbon dioxide (CO2). CH3(CH2)8COOH + NaHCO3(aq) → CH3(CH2)8COO−Na+(aq) + H2O(ℓ) + CO2(g) ↵

