Chapter 19: Organic Chemistry
Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry
by Gregory Anderson; Jen Booth; Caryn Fahey; Adrienne Richards; Samantha Sullivan Sauer; and David Wegman
Chapter 19 Contents
- 19.1 Alkanes, Alkenes, Alkynes and Aromatic Hydrocarbons
- 19.2 Alcohols and Ethers
- 19.3 Aldehydes, Ketones, Carboxylic Acids and Esters
- 19.4 Amines and Amides
- 19.5 Families of Organic Molecules – Functional Groups
- 19.6 General Reactions of Carbon
- 19.7 Introduction to Green Chemistry
- Chapter 19 – Summary
- Chapter 19 – Review
- Chapter 19 – Infographic descriptions
Except where otherwise noted, this OER is licensed under CC BY-NC-SA 4.0
Please visit the web version of Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry to access the complete book, interactive activities and ancillary resources.
In this chapter, you will learn about:
- Hydrocarbons
- Alkanes, Alkenes, Alkynes and Aromatic Hydrocarbons
- Alcohols and Ethers
- Aldehydes, Ketones, Carboxylic Acids, and Esters
- Amines and Amides
- Classifying Functional Groups
To better support your learning, you should be familiar with the following concepts before starting this chapter:
- The elements hydrogen and carbon.

All living things on earth are formed mostly of carbon compounds. The prevalence of carbon compounds in living things has led to the epithet “carbon-based” life. The truth is we know of no other kind of life. Early chemists regarded substances isolated from organisms (plants and animals) as a different type of matter that could not be synthesized artificially, and these substances were thus known as organic compounds. The widespread belief called vitalism held that organic compounds were formed by a vital force present only in living organisms. The German chemist Friedrich Wohler was one of the early chemists to refute this aspect of vitalism, when, in 1828, he reported the synthesis of urea, a component of many body fluids, from nonliving materials. Since then, it has been recognized that organic molecules obey the same natural laws as inorganic substances, and the category of organic compounds has evolved to include both natural and synthetic compounds that contain carbon. Some carbon-containing compounds are not classified as organic, for example, carbonates and cyanides, and simple oxides, such as CO and CO2. Although a single, precise definition has yet to be identified by the chemistry community, most agree that a defining trait of organic molecules is the presence of carbon as the principal element, bonded to hydrogen and other carbon atoms.
Scientists of the 18th and early 19th centuries studied compounds obtained from plants and animals and labeled them organic because they were isolated from “organized” (living) systems. Compounds isolated from nonliving systems, such as rocks and ores, the atmosphere, and the oceans, were labeled inorganic. For many years, scientists thought organic compounds could be made by only living organisms because they possessed a vital force found only in living systems.
The word organic has different meanings. Organic fertilizer, such as cow manure, is organic in the original sense; it is derived from living organisms. Organic foods generally are foods grown without synthetic pesticides or fertilizers. Organic chemistry is the chemistry of compounds of carbon. Refer to Appendix A: Key Element Information for more details about carbon and other elements.
Carbon is unique among the other elements in that its atoms can form stable covalent bonds with each other and with atoms of other elements in a multitude of variations. The resulting molecules can contain from one to millions of carbon atoms. We previously surveyed organic chemistry by dividing its compounds into families based on functional groups. We begin with the simplest members of a family and then move on to molecules that are organic in the original sense—that is, they are made by and found in living organisms. These complex molecules (all containing carbon) determine the forms and functions of living systems and are the subject of biochemistry.
Organic compounds, like inorganic compounds, obey all the natural laws. Often there is no clear distinction in the chemical or physical properties among organic and inorganic molecules. Nevertheless, it is useful to compare typical members of each class, as in Table 19.0a.
| Organic | Hexane | Inorganic | NaCl |
|---|---|---|---|
| low melting points | −95°C | high melting points | 801°C |
| low boiling points | 69°C | high boiling points | 1,413°C |
| low solubility in water; high solubility in nonpolar solvents | insoluble in water; soluble in gasoline | greater solubility in water; low solubility in nonpolar solvents | soluble in water; insoluble in gasoline |
| flammable | highly flammable | nonflammable | nonflammable |
| aqueous solutions do not conduct electricity | nonconductive | aqueous solutions conduct electricity | conductive in aqueous solution |
| exhibit covalent bonding | covalent bonds | exhibit ionic bonding | ionic bonds |
Table source: “12.1: Organic Chemistry” In Basics of GOB (Ball et al.), CC BY-NC-SA 4.0.
Keep in mind, however, that there are exceptions to every category in this table. To further illustrate typical differences among organic and inorganic compounds, Table 19a also lists properties of the inorganic compound sodium chloride (common table salt, NaCl) and the organic compound hexane (C6H14), a solvent that is used to extract soybean oil from soybeans (among other uses). Many compounds can be classified as organic or inorganic by the presence or absence of certain typical properties, as illustrated in Table 19a.
The largest database[1] of organic compounds lists about 10 million substances, which include compounds originating from living organisms and those synthesized by chemists. The number of potential organic compounds has been estimated[2] at 1060—an astronomically high number. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to four strong bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities.
The simplest organic compounds contain only the elements carbon and hydrogen, and are called hydrocarbons. Even though they are composed of only two types of atoms, there is a wide variety of hydrocarbons because they may consist of varying lengths of chains, branched chains, and rings of carbon atoms, or combinations of these structures. In addition, hydrocarbons may differ in the types of carbon-carbon bonds present in their molecules. Many hydrocarbons are found in plants, animals, and their fossils; other hydrocarbons have been prepared in the laboratory. We use hydrocarbons every day, mainly as fuels, such as natural gas, acetylene, propane, butane, and the principal components of gasoline, diesel fuel, and heating oil. The familiar plastics polyethylene, polypropylene, and polystyrene are also hydrocarbons. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. This leads to differences in geometries and in the hybridization of the carbon orbitals.
As mentioned above, hydrocarbons can be man-made (in the laboratory) or naturally present. How do we know which is better for us overall? Well, it depends on the organic compound in question. Both man-made and natural products can be good or bad for us. Infographic 19.0a looks at some common misconceptions about man-made and natural chemicals.
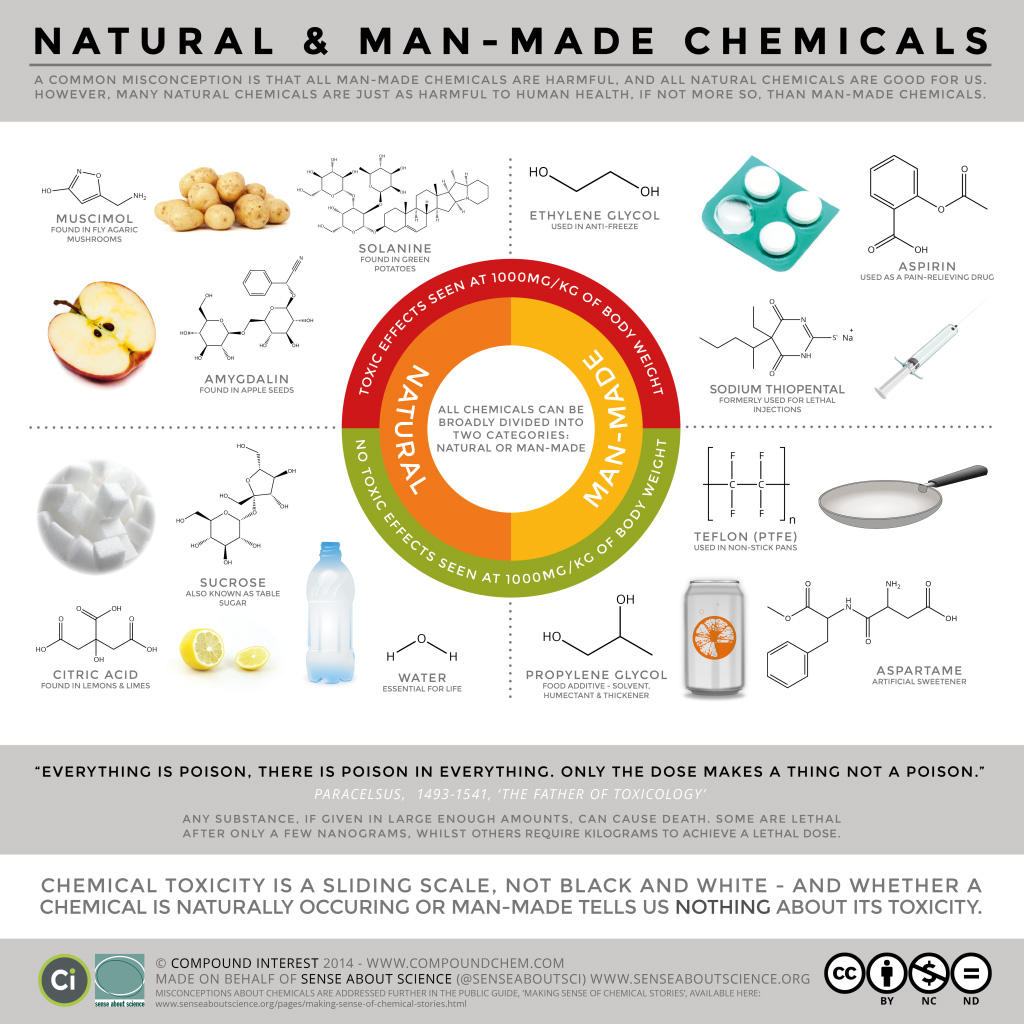
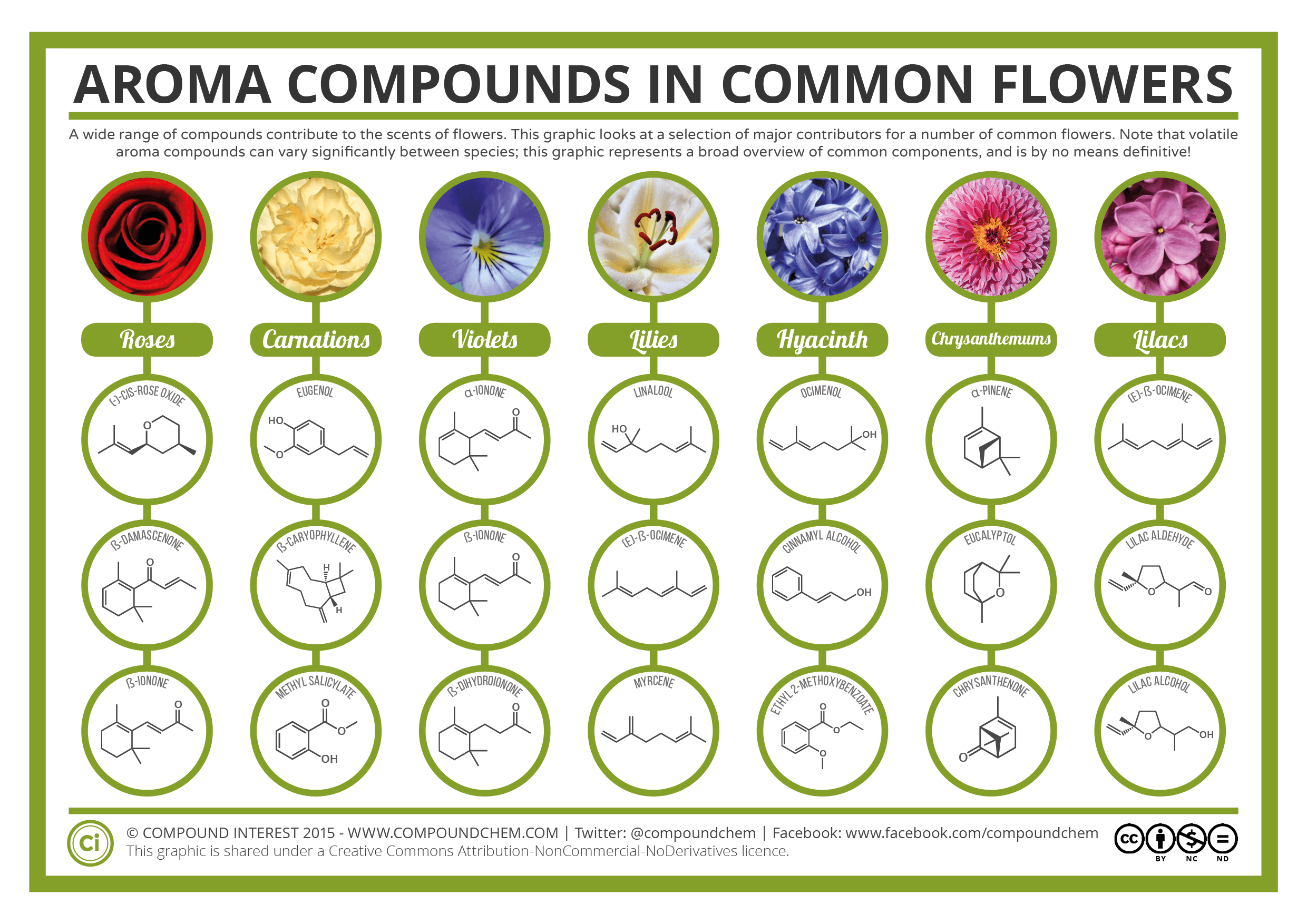
Today, organic compounds are key components of plastics, soaps, perfumes, sweeteners, fabrics, pharmaceuticals, and many other substances that we use every day. The value to us of organic compounds ensures that organic chemistry is an important discipline within the general field of chemistry. In this chapter, we discuss why the element carbon gives rise to a vast number and variety of compounds, how those compounds are classified, and the role of organic compounds in representative biological and industrial settings. Infographic 19.0b looks at one of the roles organic compounds play in our everyday lives such as the beautiful aromas from various flowers.
Spotlight on Everyday Chemistry: The Scent of Flowers
The infographic 19.0b below demonstrates one of the many interesting characteristics of organic compounds. The specific aroma of some common flowers is due to the organic compound that makes up the flower.
The video below gives a summary of organic chemistry and the upcoming topics that will be described in this textbook.
Watch Crash Course Organic Chemistry Preview (3 mins) on YouTube
Video source: CrashCourse. (2020, April 22). Crash Course organic chemistry preview [Video]. YouTube.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from the following sources:
- “Chapter 18 Introduction“, “18.1 Hydrocarbons” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax) and
- “12.1: Organic Chemistry” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- This is the Beilstein database, now available through the Reaxys site . ↵
- Peplow, Mark. “Organic Synthesis: The Robo-Chemist,” Nature 512 (2014): 20–2. ↵
natural or synthetic compound that contains carbon

