Chapter 22: Alkenes, Alkynes and Aromatics
Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry
by Gregory Anderson; Jen Booth; Caryn Fahey; Adrienne Richards; Samantha Sullivan Sauer; and David Wegman
Chapter 22 Contents
- 22.1 Alkenes and Alkynes – Structure and Naming
- 22.2 Structure of Alkenes – Cis-Trans Isomers
- 22.3 Reactions of Alkenes and Alkynes
- 22.4 Aromatic Compounds – Structure and Naming
- 22.5 Aromatic Reactions
- Chapter 22 – Summary
- Chapter 22 – Review
- Chapter 22 – Infographic descriptions
Except where otherwise noted, this OER is licensed under CC BY-NC-SA 4.0
Please visit the web version of Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry to access the complete book, interactive activities and ancillary resources.
In this chapter, you will learn about:
- Naming and drawing unsaturated hydrocarbons
- Identifying cis-trans isomers
- Formation and use of unsaturated hydrocarbons
To better support your learning, you should be familiar with the following concepts before starting this chapter:
- Alkene, alkyne and aromatic functional groups (Chapter 22 Alkenes, Alkynes and Aromatics)
- Alkane naming and drawing (Chapter 19 Alkanes and Alkyl Halides)
- General reactions of carbon (Chapter 19.6 General Reactions of Carbon)
- Advanced bonding of carbon (Chapter 21 Advanced theories of Covalent Bonding)
Our modern society is based to a large degree on the chemicals we discuss in this chapter. Most are made from petroleum. Alkanes—saturated hydrocarbons—have relatively few important chemical properties other than that they undergo combustion and react with halogens. Unsaturated hydrocarbons—hydrocarbons with double or triple bonds—on the other hand, are quite reactive. In fact, they serve as building blocks for many familiar plastics—polyethylene, vinyl plastics, acrylics—and other important synthetic materials (e.g., alcohols, antifreeze, and detergents). Aromatic hydrocarbons have formulas that can be drawn as cyclic alkenes, making them appear unsaturated, but their structure and properties are generally quite different, so they are not considered to be alkenes. Aromatic compounds serve as the basis for many drugs, antiseptics, explosives, solvents, and plastics (e.g., polyesters and polystyrene).

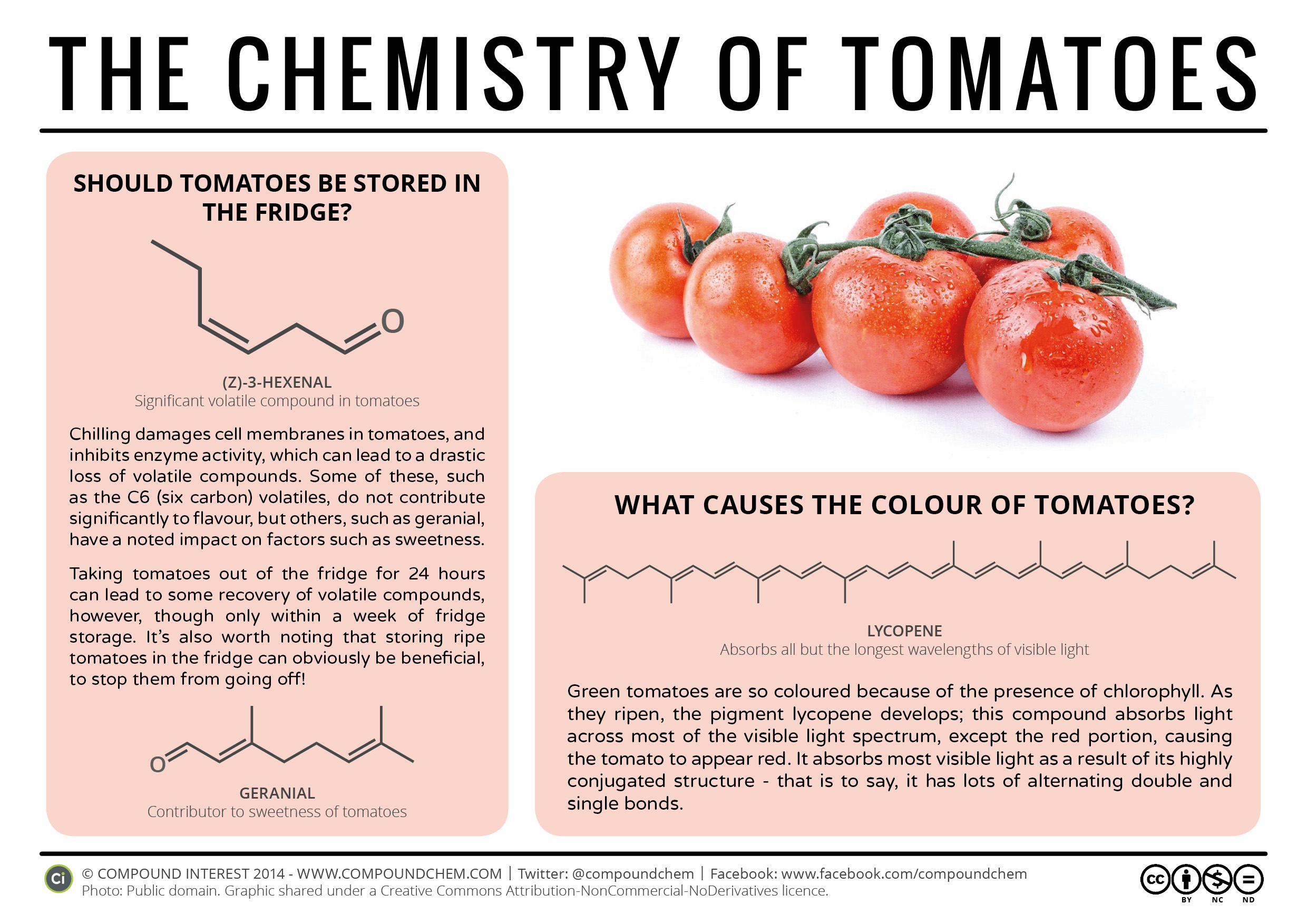
Alkenes also occur widely in nature. Ripening fruits and vegetables give off ethylene, which triggers further ripening. Fruit processors artificially introduce ethylene to hasten the ripening process; exposure to as little as 0.1 mg of ethylene for 24 h can ripen 1 kg of tomatoes as in Figure 22.0a. Unfortunately, this process does not exactly duplicate the ripening process, and tomatoes picked green and treated this way don’t taste much like vine-ripened tomatoes fresh from the garden. The bright red colour of tomatoes is due to lycopene—a polyene (Figure 22.0b.).
Other alkenes that occur in nature include 1-octene, a constituent of lemon oil, and octadecene (C18H36) found in fish liver. Dienes (two double bonds) and polyenes (three or more double bonds) are also common. Butadiene (CH2=CHCH=CH2) is found in coffee. Lycopene and the carotenes are isomeric polyenes (C40H56) that give the attractive red, orange, and yellow colours to watermelons, tomatoes, carrots, and other fruits and vegetables. Vitamin A, essential to good vision, is derived from a carotene. The world would be a much less colourful place without alkenes. Infographic 22.0a. discusses if tomatoes should be stored in the fridge.
Attribution & References
Except where otherwise noted, this page is adapted by David Wegman, Adrienne Richards and Samantha Sullivan Sauer from
- “13.4: Properties of Alkenes and Alkynes” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0 . / A derivative of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
- 1st paragraph is adapted from “13.0: Prelude to Unsaturated and Aromatic Hydrocarbons” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0

