22.2 Structure of Alkenes – Cis-Trans Isomers
Learning Objectives
By the end of this section, you will be able to:
- Recognize that alkenes that can exist as cis-trans isomers.
- Classify isomers as cis or trans.
- Draw structures for cis-trans isomers given their names.
There is free rotation about the carbon-to-carbon single bonds (C–C) in alkanes. In contrast, the structure of alkenes requires that the carbon atoms of a double bond and the two atoms bonded to each carbon atom all lie in a single plane, and that each doubly bonded carbon atom lies in the center of a triangle. This part of the molecule’s structure is rigid; rotation about doubly bonded carbon atoms is not possible without rupturing the bond. Look at the two chlorinated hydrocarbons in Figure 22.2a.
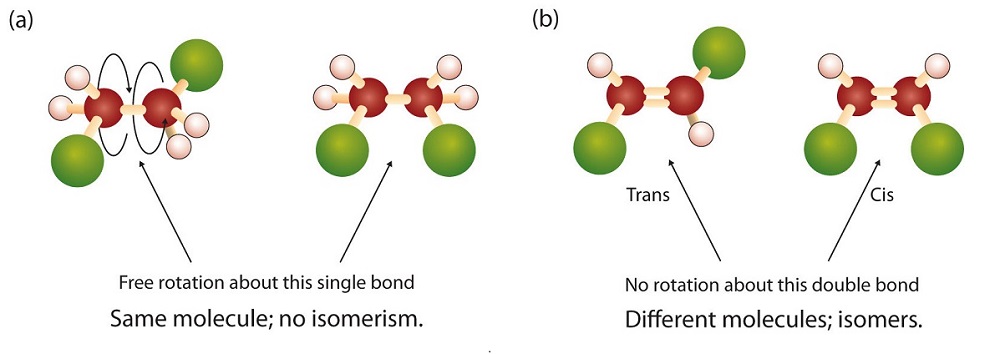
In 1,2-dichloroethane (part (a) of Figure 22.2b.), there is free rotation about the C–C bond. The two models shown represent exactly the same molecule; they are not isomers. You can draw structural formulas that look different, but if you bear in mind the possibility of this free rotation about single bonds, you should recognize that these two structures represent the same molecule:

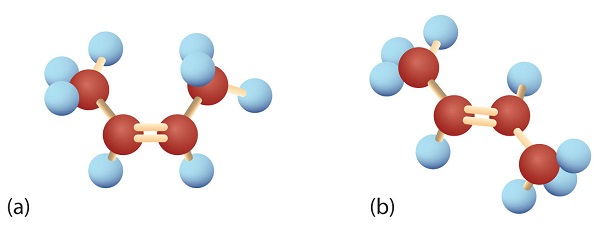
In 1,2-dichloroethene (part (b) of Figure 22.2b.), however, restricted rotation about the double bond means that the relative positions of substituent groups above or below the double bond become significant. This leads to a special kind of isomerism. The isomer in which the two chlorine (Cl) atoms lie on the same side of the molecule is called the cis isomer (Latin cis, meaning “on this side”) and is named cis-1,2-dichloroethene. The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer (Latin trans, meaning “across”) and is named trans-1,2-dichloroethene. These two compounds are cis-trans isomers (or geometric isomers), compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule.
Consider the alkene with the condensed structural formula CH3CH=CHCH3. We could name it 2-butene, but there are actually two such compounds; the double bond results in cis-trans isomerism (Figure 22.2c.).
Cis-2-butene has both methyl groups on the same side of the molecule. Trans-2-butene has the methyl groups on opposite sides of the molecule. Their structural formulas are as follows according to Figure 22.2d.

Note, however, that the presence of a double bond does not necessarily lead to cis-trans isomerism (Figure 22.2e.). We can draw two seemingly different propenes:

However, these two structures are not really different from each other. If you could pick up either molecule from the page and flip it over top to bottom, you would see that the two formulas are identical. Thus, there are two requirements for cis-trans isomerism:
- Rotation must be restricted in the molecule.
- There must be two nonidentical groups on each doubly bonded carbon atom.
In these propene structures, the second requirement for cis-trans isomerism is not fulfilled. One of the doubly bonded carbon atoms does have two different groups attached, but the rules require that both carbon atoms have two different groups. In general, the following statements hold true in cis-trans isomerism:
- Alkenes with a C=CH2 unit do not exist as cis-trans isomers.
- Alkenes with a C=CR2 unit, where the two R groups are the same, do not exist as cis-trans isomers.
- Alkenes of the type R–CH=CH–R can exist as cis and trans isomers; cis if the two R groups are on the same side of the carbon-to-carbon double bond, and trans if the two R groups are on opposite sides of the carbon-to-carbon double bond.
Advanced Note: E/Z Isomerization
If a molecule has a C=C bond with one non-hydrogen group attached to each of the carbons, cis/trans nomenclature described above is enough to describe it. However, if you have three different groups (or four), then the cis/trans approach is insufficient to describe the different isomers, since we do not know which two of the three groups are being described. For example, if you have a C=C bond, with a methyl group and a bromine on one carbon, and an ethyl group on the other, it is neither trans nor cis, since it is not clear whether the ethyl group is trans to the bromine or the methyl.
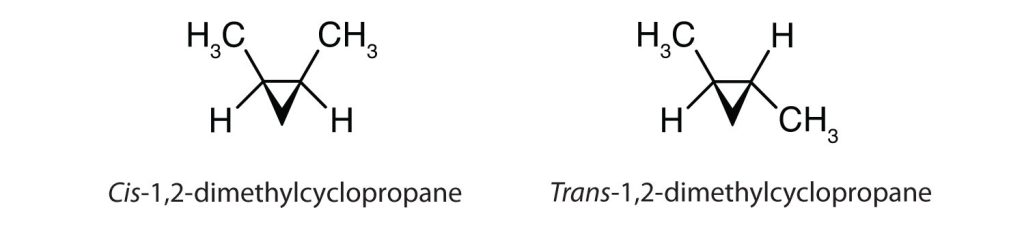
Cis-trans isomerism also occurs in cyclic compounds. In ring structures, groups are unable to rotate about any of the ring carbon–carbon bonds. Therefore, groups can be either on the same side of the ring (cis) or on opposite sides of the ring (trans). For our purposes here, we represent all cycloalkanes as planar structures, and we indicate the positions of the groups, either above or below the plane of the ring. Refer to Figure 22.2f. to compare cis-1,2-dimethylcyclopropane and trans-1,2-dimethylcyclopropane.
For a look at the types of isomerism in organic chemistry. Refer back to Infographic 20.3a.
Which compounds can exist as cis-trans (geometric) isomers? Draw them.
- CHCl=CHBr
- CH2=CBrCH3
- (CH3)2C=CHCH2CH3
- CH3CH=CHCH2CH3
Solution
All four structures have a double bond and thus meet rule 1 for cis-trans isomerism.
- This compound meets rule 2; it has two nonidentical groups on each carbon atom (H and Cl on one and H and Br on the other). It exists as both cis and trans isomers:

(Credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
- This compound has two hydrogen atoms on one of its doubly bonded carbon atoms; it fails rule 2 and does not exist as cis and trans isomers.
- This compound has two methyl (CH3) groups on one of its doubly bonded carbon atoms. It fails rule 2 and does not exist as cis and trans isomers.
- This compound meets rule 2; it has two nonidentical groups on each carbon atom and exists as both cis and trans isomers:

Exercise 22.2a
Which compounds can exist as cis-trans isomers?
a. CH2=CHCH2CH2CH3
b. CH3CH=CHCH2CH3
c. CH3CH2CH=CHCH2CH3
d.
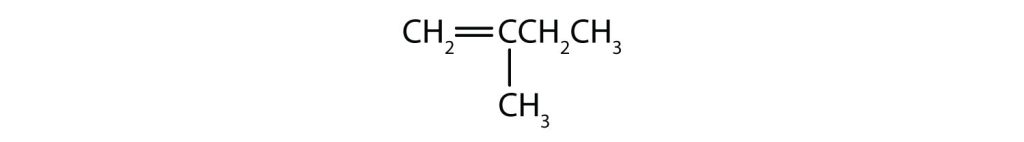
e.
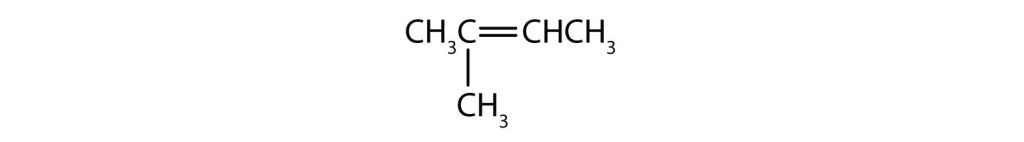
Check Your Answers:[1]
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards and Samantha Sullivan Sauer from “13.3: The Structure of Alkenes- Cis-Trans Isomerism” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.) , CC BY-NC-SA 3.0. / A derivative of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
- a) no cis-trans isomers b) cis-trans isomers are possible c) cis-trans isomers are possible d) no cis-trans isomers e) no cis-trans isomers ↵

