27.1 Polymerization
Learning Objectives
By the end of this section, you will be able to:
- Define the terms monomer and polymer.
- Know some different examples of synthetic and natural polymers.
A polymer is a large molecule, or macromolecule, composed of many repeated subunits. The term “polymer” derives from the Greek word polus (meaning “many, much”) and meros (meaning “part”) and refers to a molecule whose structure is composed of multiple repeating units, from which originates a characteristic of high relative molecular mass and attendant properties. An example of a polymer is shown in Figure 27.1a.
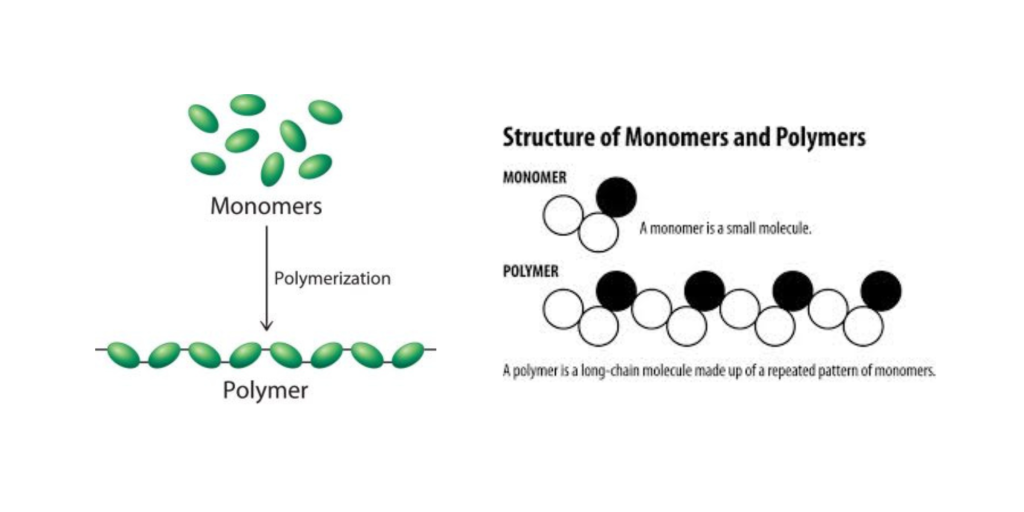
Due to their broad range of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass relative to small molecule compounds produces unique physical properties, including toughness, viscoelasticity, and a tendency to form glasses and semicrystalline structures rather than crystals. The terms polymer and resin are often synonymous with plastic.
Natural Polymers
Some very important biological materials are polymers. Of the three major food groups, polymers are represented in two: proteins and carbohydrates. Proteins are polymers of amino acids, which are monomers that have an amine functional group and a carboxylic acid functional group. Proteins play a crucial role in living organisms.
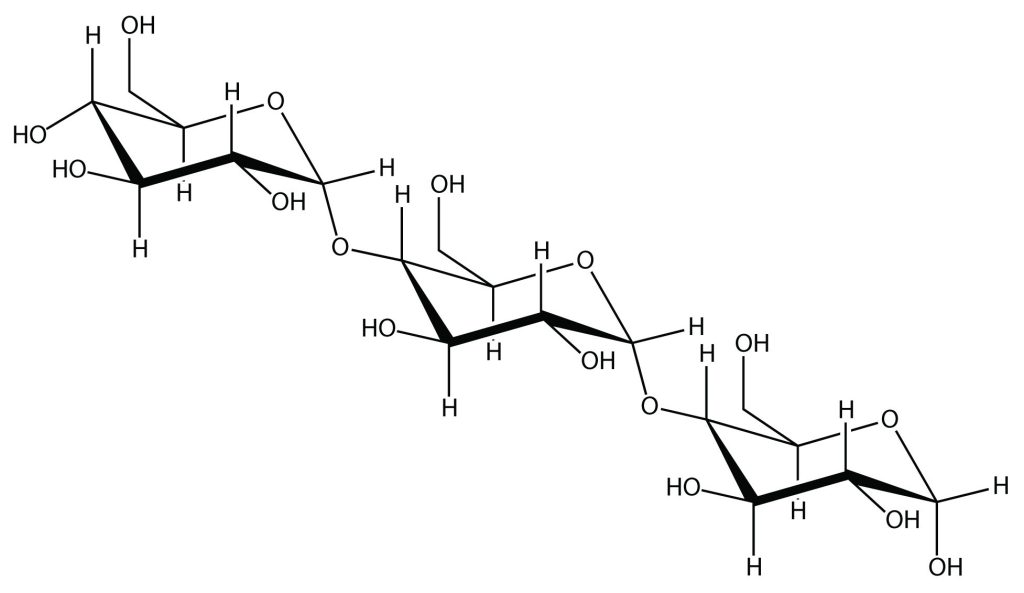
Another example of a natural polymer involves linking hundreds of glucose molecules together to make a relatively common material known as starch. Starch is an important source of energy in the human diet. Note, in Figure 27.1b., how the individual glucose units are joined together to form starch.
Glucose molecules can also be joined together in another way, as shown in Figure 27.1c., to form a polymer known as cellulose. Cellulose forms the strands found in cotton that we use in clothing.
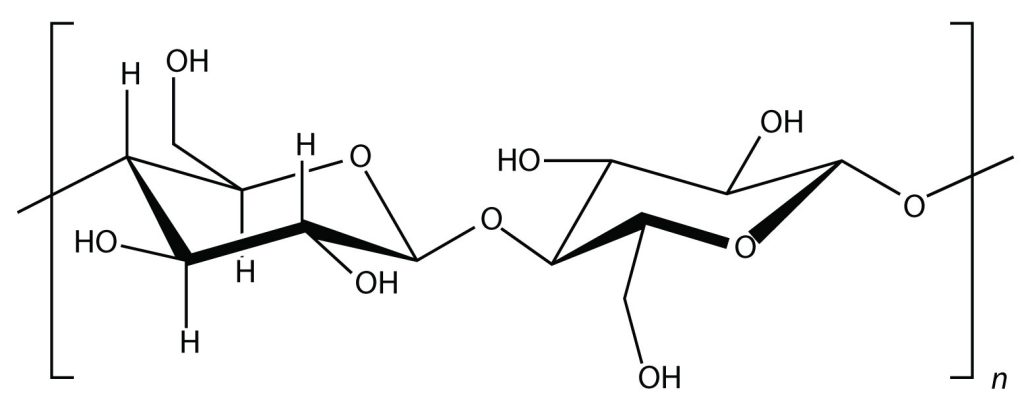
Cellulose is a major component in the cell walls of plants. Curiously, despite the similarity in the building blocks between starch and cellulose, some animals (such as humans) cannot digest cellulose; those animals that can digest cellulose typically rely on symbiotic bacteria in the digestive tract for the actual digestion. Animals do not have the proper enzymes to break apart the glucose units in cellulose, so it passes through the digestive tract and is considered dietary fiber.
Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) are also polymers, composed of long, three-part chains consisting of phosphate groups, sugars with 5 C atoms (ribose or deoxyribose), and N-containing rings referred to as bases. Each combination of the three parts is called a nucleotide; DNA and RNA are essentially polymers of nucleotides that have rather complicated but intriguing structures (Figure 27.1d.). DNA is the fundamental material in chromosomes and is directly responsible for heredity, while RNA is an essential substance in protein synthesis. These natural polymers, or biopolymers (polymers produced by living organisms), are discussed further in Chapter 28.

Synthetic Polymers
Synthetic polymers are often formed from monomers derived from fossil fuels and petroleum products. Current research is focused on finding other more renewable sources of monomers.
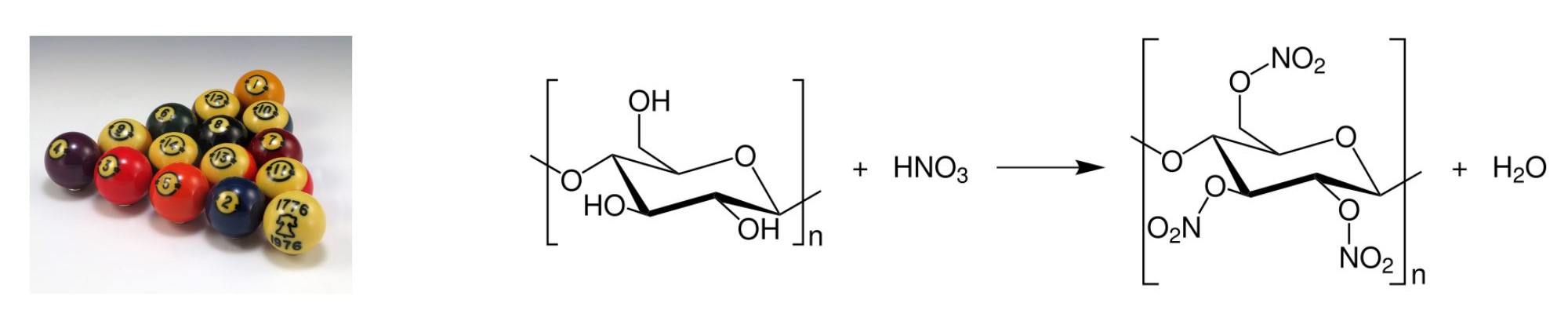
Celluloid: Billiard Balls
Celluloids are a class of compounds created from nitrocellulose (partially nitrated cellulose) and camphor, with added dyes and other agents. Generally considered the first thermoplastic, it was first created as Parkesinein (by Alexander Parkes of Birmingham England) in 1856 and as Xylonite in 1869. In the 1860s, an American, John Wesley Hyatt, acquired Parkes’s patent and began experimenting with cellulose nitrate with the intention of manufacturing billiard balls, which until that time were made from ivory. In the 1870s the modified plastic was registered as “celluloid”. The formation and structure of celluloid is shown in Figure 27.1e.
The main use was in movie and photography film industries, which used only celluloid film stock prior to the adoption of acetate safety film in the 1950s. Celluloid is highly flammable, difficult and expensive to produce and no longer widely used; its most common uses today are in table tennis balls, musical instruments, and guitar picks.
Bakelite
Bakelite (sometimes spelled Baekelite) or polyoxybenzylmethylenglycolanhydride was the first plastic made from synthetic components. It is a thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. It was developed by the Belgian-American chemist Leo Baekeland in Yonkers, New York, in 1907.
Bakelite was patented on December 7, 1909. The creation of a synthetic plastic was revolutionary for its electrical non conductivity and heat-resistant properties in electrical insulators, radio and telephone casings and such diverse products as kitchenware, jewelry, pipe stems, children’s toys, and firearms. Figure 27.1f. shows examples of products made from Bakelite.

Watch Polymers: Crash Course Chemistry #45 – YouTube (10 min)
Video source: Crash Course. (2014, January 6). Polymers: Crash Course Chemistry #45 – YouTube [Video]. YouTube.
Attribution & References
Except where otherwise noted, this page has been adapted by Samantha Sullivan Sauer from
- “10.1: Polymerization – Making Big Ones Out of Little Ones” In Map: Chemistry for Changing Times (Hill and McCreary) by LibreTexts, licensed under CC BY-SA .
Contributors from original source:- Joshua Halpern, Scott Sinex and Scott Johnson, Marisa Alviar-Agnew (Sacramento City College)
- Beginning Chemistry (Ball), CC BY-NC-SA 3.0, a LibreTexts version of Beginning Chemistry (v 1.0).
- Wikipedia
large molecule, or macromolecule, composed of many repeated subunits
a very large molecule containing thousands of atoms covalently bonded together in a specific structure
man-made
found in nature
Process of monomers bonding together to form a polymer
smallest repeating unit that links together to form a polymer
polymers of amino acids, which are monomers that have an amine functional group and a carboxylic acid functional group
important source of energy in the human diet formed from repeating glucose units
major component in the cell walls of plants formed from repeating units of glucose
polymers, composed of long, three-part chains consisting of phosphate groups, sugars with 5 C atoms (ribose or deoxyribose), and N-containing rings referred to as bases
class of compounds created from nitrocellulose (partially nitrated cellulose) and camphor, with added dyes and other agents

