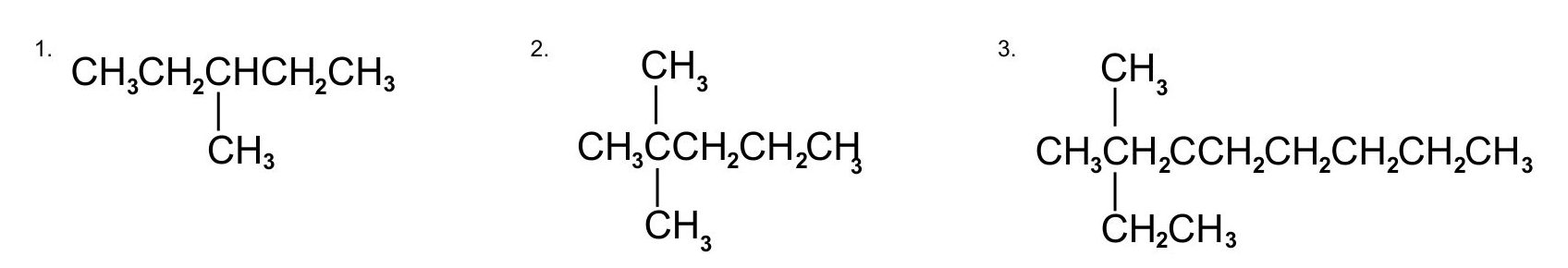20.3 Isomers of Alkanes and IUPAC Nomenclature
Learning Objectives
By the end of this section, you will be able to:
- Identify simple alkanes as straight-chain or branched-chain.
- Describe and recognize structural and functional group isomers.
- Name alkanes by the IUPAC system and write formulas for alkanes given IUPAC names
Isomers
Hydrocarbons with the same formula, including alkanes, can have different structures. For example, two alkanes have the formula C4H10: They are called n-butane and 2-methylpropane (or isobutane), and have the following structural formulas as shown in Figure 20.3a:
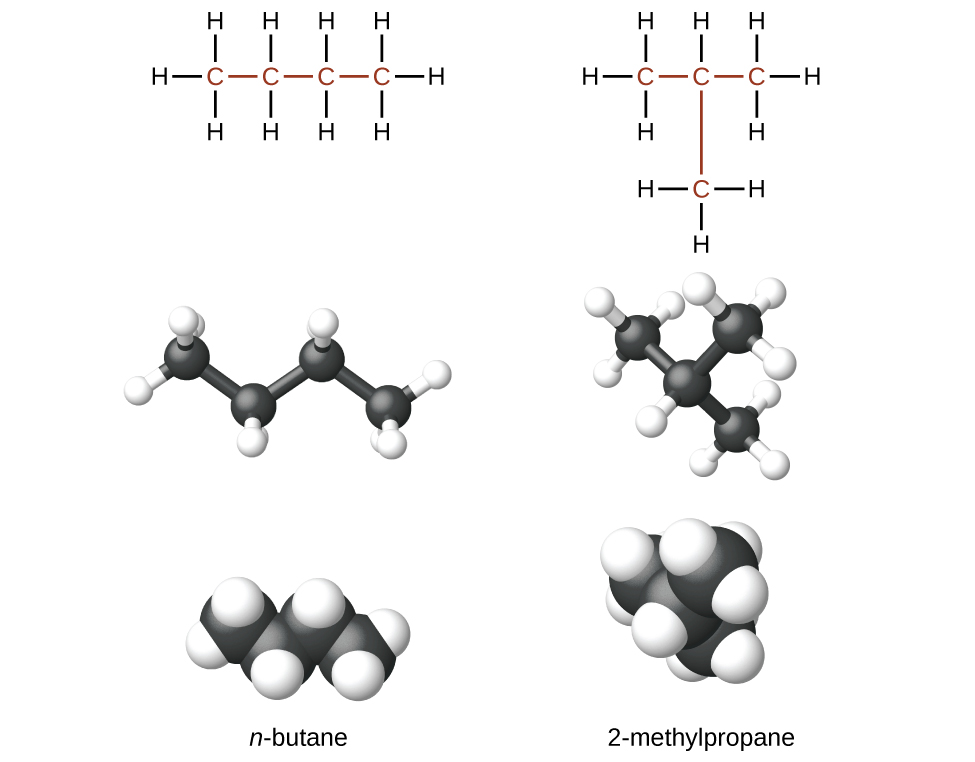
The compounds n-butane and 2-methylpropane are structural isomers (the term constitutional isomers is also commonly used). Constitutional isomers have the same molecular formula but different spatial arrangements of the atoms in their molecules. The n-butane molecule contains an unbranched chain, meaning that no carbon atom is bonded to more than two other carbon atoms. We use the term normal, or the prefix n, to refer to a chain of carbon atoms without branching. The compound 2–methylpropane has a branched chain (the carbon atom in the center of the structural formula is bonded to three other carbon atoms).
Recall from section 20.2, Table 20.3a. which shows the molecular formula and condensed structural formulas for the first 10 straight-chain alkanes. Table 20.3a also shows the number of isomers for each alkane. The number of isomers increases rapidly as the number of carbon atoms increases.
| Name | Molecular Formula (CnH2n + 2) | Condensed Structural Formula | Number of Possible Isomers |
|---|---|---|---|
| methane | CH4 | CH4 | — |
| ethane | C2H6 | CH3CH3 | — |
| propane | C3H8 | CH3CH2CH3 | — |
| butane | C4H10 | CH3CH2CH2CH3 | 2 |
| pentane | C5H12 | CH3CH2CH2CH2CH3 | 3 |
| hexane | C6H14 | CH3CH2CH2CH2CH2CH3 | 5 |
| heptane | C7H16 | CH3CH2CH2CH2CH2CH2CH3 | 9 |
| octane | C8H18 | CH3CH2CH2CH2CH2CH2CH2CH3 | 18 |
| nonane | C9H20 | CH3CH2CH2CH2CH2CH2CH2CH2CH3 | 35 |
| decane | C10H22 | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 | 75 |
Table source: “12.2: Structures and Names of Alkanes” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
Identifying isomers from structural formulae is not as easy as it looks. Structural formulae that look different may actually represent the same isomers. For example, the three structures in Figure 20.3b all represent the same molecule, n-butane, and hence are not different isomers. They are identical because each contains an unbranched chain of four carbon atoms.
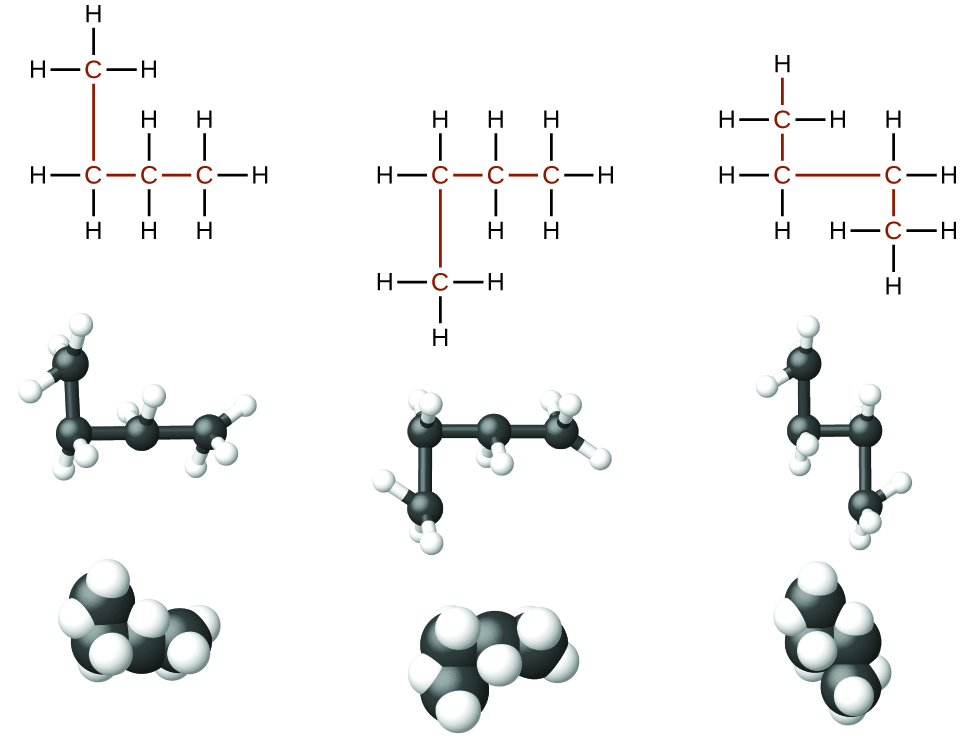
When identifying isomers, it is useful to trace the carbon backbone with your finger or a pencil and count carbons until you need to lift your hand or pencil to get the another carbon. Try this with each of the above arrangements of four carbons above in Figure 20.3b. Butane has a continuous chain of four carbons no matter how the bonds are rotated – you can connect the carbons in a line without lifting your finger from the page. In a later portion of this chapter, you will learn how to systematically name compounds by counting the number of carbons in the longest continuous chain and identifying any functional groups present.
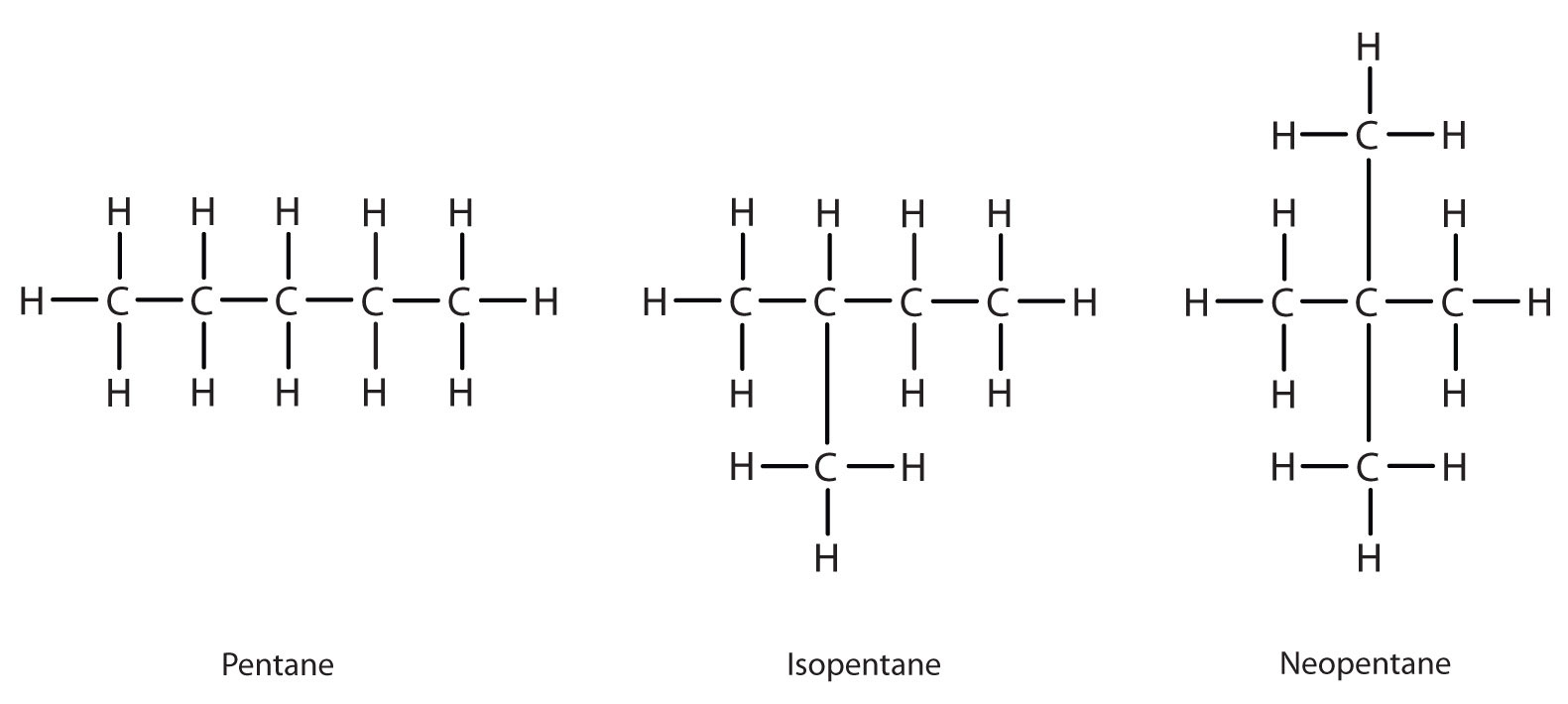
Adding one more carbon to the butane chain gives pentane, which has the formula, C5H12. Pentane and its two branched-chain isomers are shown below in Figure 20.3c. The compound at the far left is pentane because it has all five carbon atoms in a continuous chain. The compound in the middle is isopentane; like isobutane, it has a one CH3 branch off the second carbon atom of the continuous chain. The compound at the far right, discovered after the other two, was named neopentane (from the Greek neos, meaning “new”). Although all three have the same molecular formula, they have different properties, including boiling points: pentane, 36.1°C; isopentane, 27.7°C; and neopentane, 9.5°C. The names isopentane and neopentane are common names for these molecules. As mentioned above, we will learn the systematic rules for naming compounds next.
IUPAC System of Nomenclature for Alkanes
Looking at Table 20.3a., there are 3 pentanes, 5 hexanes, 9 heptanes, and 18 octanes. It would be difficult to assign unique individual names that we could remember. A systematic way of naming hydrocarbons and other organic compounds has been devised by the International Union of Pure and Applied Chemistry (IUPAC). These rules, used worldwide, are known as the IUPAC System of Nomenclature. A stem name (Table 20.3b.) indicates the number of carbon atoms in the longest continuous chain (LCC). Atoms or groups attached to this carbon chain, called substituents, are then named, with their positions indicated by numbers. For now, we will consider only those substituents called alkyl groups.
| Stem | Number |
|---|---|
| meth- | 1 |
| eth- | 2 |
| prop- | 3 |
| but- | 4 |
| pent- | 5 |
| hex- | 6 |
| hept- | 7 |
| oct- | 8 |
| non- | 9 |
| dec- | 10 |
Table source: “12.5: IUPAC Nomenclature” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
An alkyl group is a group of atoms that results when one hydrogen atom is removed from an alkane. The group is named by replacing the -ane suffix of the parent hydrocarbon with -yl. For example, the -CH3 group derived from methane (CH4) results from subtracting one hydrogen atom and is called a methyl group. The alkyl groups we will use most frequently are listed in Table 20.3c. Alkyl groups are not independent molecules; they are parts of molecules that we consider as a unit to name compounds systematically.
| Parent Alkane | Alkyl Group | Condensed Structural Formula | ||
|---|---|---|---|---|
| methane | 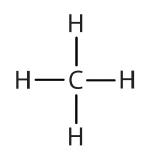 |
methyl | 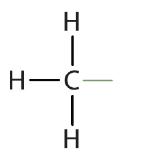 |
CH3– |
| ethane | 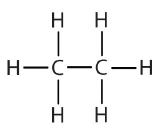 |
ethyl | 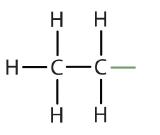 |
CH3CH2– |
| propane | 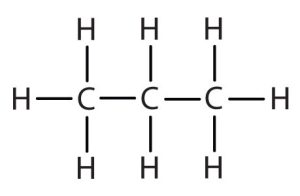 |
propyl | 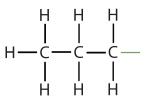 |
CH3CH2CH2– |
| isopropyl | 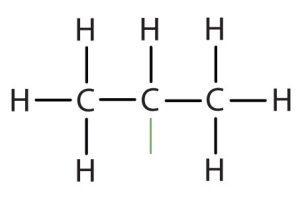 |
(CH3)2CH– | ||
| butane | 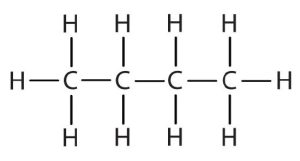 |
butyl* | 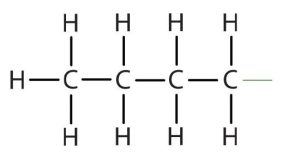 |
CH3CH2CH2CH2– |
Table 20.3c. note: *There are four butyl groups, two derived from butane and two from isobutane. We will introduce the other three where appropriate. (Image credits: Introduction to Chemistry: GOB (V. 1.0). , CC BY-NC-SA 3.0).
Simplified IUPAC rules for naming alkanes are as follows (demonstrated in Example 20.3a.).
- Name alkanes according to the LCC (longest continuous chain) of carbon atoms in the molecule (rather than the total number of carbon atoms). This LCC, considered the parent chain, determines the base name, to which we add the suffix –ane to indicate that the molecule is an alkane.
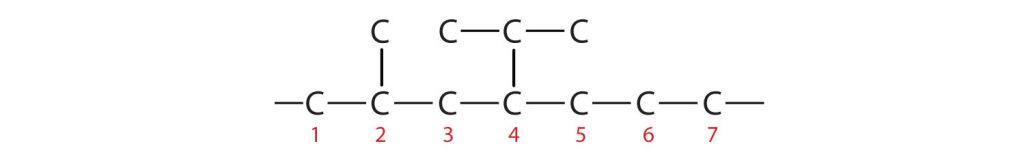
- If the hydrocarbon is branched, number the carbon atoms of the LCC. Numbers are assigned in the direction that gives the lowest numbers to the carbon atoms with attached substituents. Hyphens are used to separate numbers from the names of substituents; commas separate numbers from each other. (The LCC need not be written in a straight line; for example, the LCC in the following has five carbon atoms.)
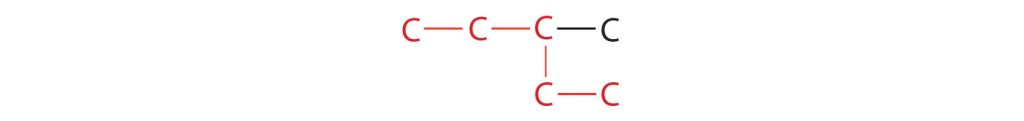
Figure 20.3d. An example showing that finding the longest carbon chain does not have to be in a straight line. (Credit: Introduction to Chemistry: GOB (V. 1.0). ,CC BY-NC-SA 3.0.) - Place the names of the substituent groups in alphabetical order before the name of the parent compound. If the same alkyl group appears more than once, the numbers of all the carbon atoms to which it is attached are expressed. If the same group appears more than once on the same carbon atom, the number of that carbon atom is repeated as many times as the group appears. Moreover, the number of identical groups is indicated by the Greek prefixes di-, tri-, tetra-, and so on. These prefixes are not considered in determining the alphabetical order of the substituents. For example, ethyl is listed before dimethyl; the di- is simply ignored. The last alkyl group named is prefixed to the name of the parent alkane to form one word.
When these rules are followed, every unique compound receives its own exclusive name. The rules enable us to not only name a compound from a given structure but also draw a structure from a given name. The best way to learn how to use the IUPAC system is to put it to work, not just memorize the rules. It’s easier than it looks.
Example 20.3a
Name the molecule whose structure is shown here:
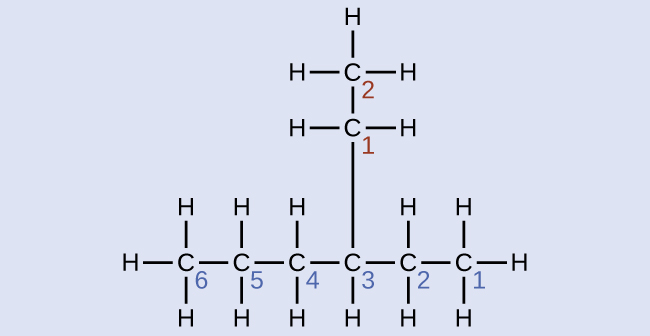
Solution
The longest carbon chain runs horizontally across the page and contains six carbon atoms (this makes the base of the name hexane, but we will also need to incorporate the name of the branch). In this case, we want to number from right to left (as shown by the blue numbers) so the branch is connected to carbon 3 (imagine the numbers from left to right—this would put the branch on carbon 4, violating our rules). The branch attached to position 3 of our chain contains two carbon atoms (numbered in red)—so we take our name for two carbons eth- and attach -yl at the end to signify we are describing a branch. Putting all the pieces together, this molecule is 3-ethylhexane.
Example & image source: General Chemistry 1 & 2 , CC BY 4.0.
Exercise 20.3a
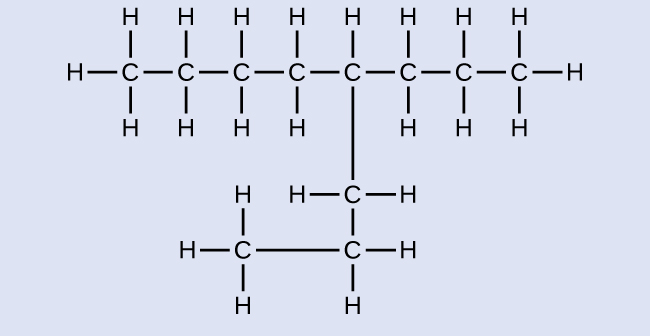
Check Your Answer[1]
Example 20.3b
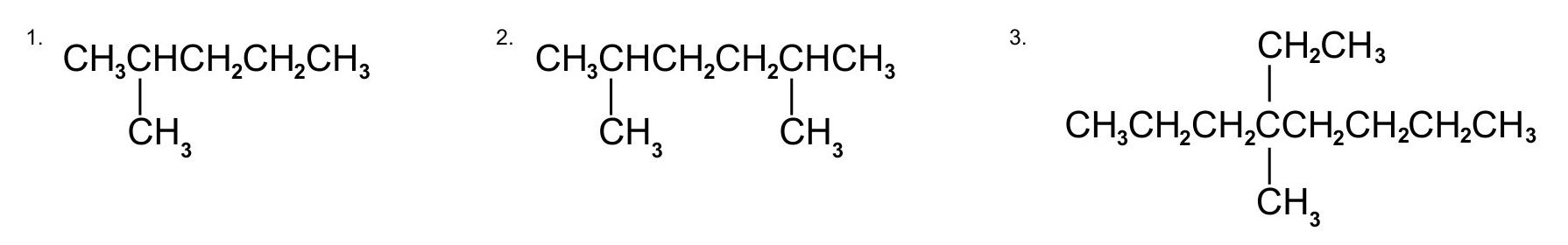
Name each compound:
Solution
- The LCC has five carbon atoms, and so the parent compound is pentane (rule 1). There is a methyl group (rule 2) attached to the second carbon atom of the pentane chain. The name is therefore 2-methylpentane.
- The LCC has six carbon atoms, so the parent compound is hexane (rule 1). Methyl groups (rule 2) are attached to the second and fifth carbon atoms. The name is 2,5-dimethylhexane.
- The LCC has eight carbon atoms, so the parent compound is octane (rule 1). There are methyl and ethyl groups (rule 2), both attached to the fourth carbon atom (counting from the right gives this carbon atom a lower number; rule 3). The correct name is thus 4-ethyl-4-methyloctane.
Example & image source: Introduction to Chemistry: GOB (V. 1.0)., CC BY-NC-SA 3.0
Exercise 20.3b
Name each compound.
Check your answer[2]
Exercise & image source: Introduction to Chemistry: GOB (V. 1.0)., CC BY-NC-SA 3.0
Draw the structure for each compound.
- 2,3-dimethylbutane
- 4-ethyl-2-methylheptane
Solution
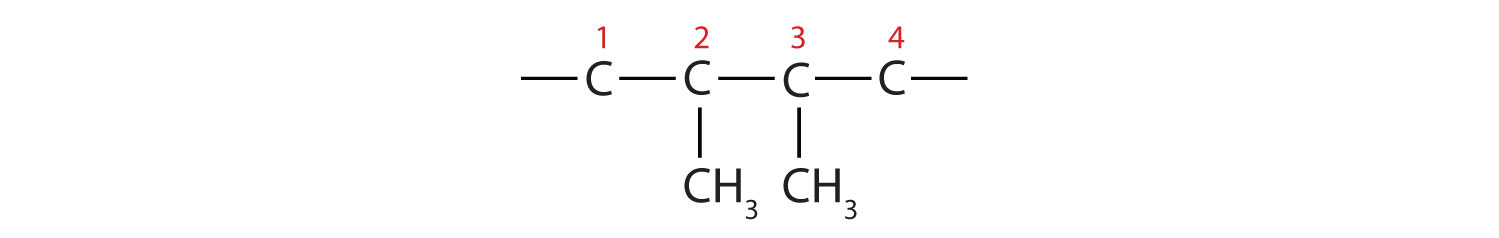
In drawing structures, always start with the parent chain.
The parent chain is butane, indicating four carbon atoms in the LCC.
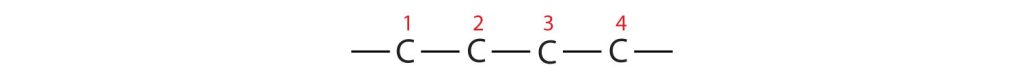
Then add the groups at their proper positions. You can number the parent chain from either direction as long as you are consistent; just don’t change directions before the structure is done. The name indicates two methyl (CH3) groups, one on the second carbon atom and one on the third.
Finally, fill in all the hydrogen atoms, keeping in mind that each carbon atom must have four bonds.
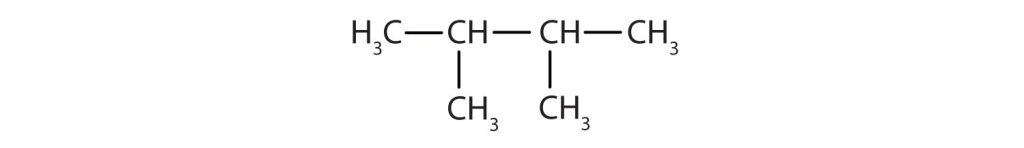
The parent chain is heptane in this case, indicating seven carbon atoms in the LCC. –C–C–C–C–C–C–C–
Adding the groups at their proper positions gives
Filling in all the hydrogen atoms gives the following condensed structural formulas:
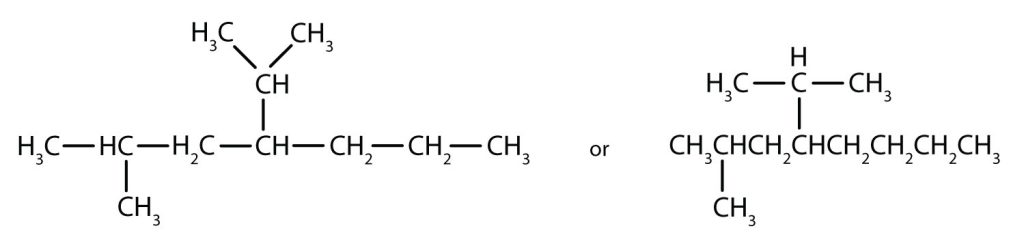
Note that the bonds (dashes) can be shown or not; sometimes they are needed for spacing.
Example & image source: Introduction to Chemistry: GOB (V. 1.0)., CC BY-NC-SA 3.0
Exercise 20.3c
Draw the structure for each compound.
4-ethyloctane
- 3-ethyl-2-methylpentane
- 3,3,5-trimethylheptane
Check your answer [3]
Want more practice naming alkanes?
Watch the video tutorial Naming simple alkanes (10 mins) on YouTube to review the nomenclature process.
Video source: Khan Academy. (2010, July 21) Naming simple alkanes [Video]. YouTube.
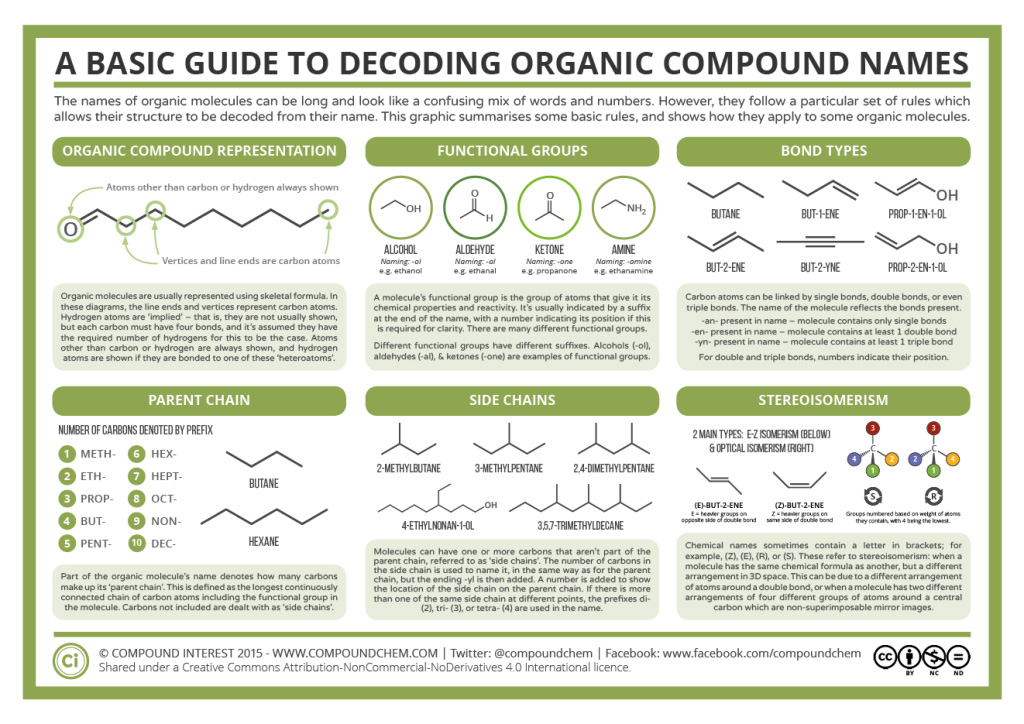
For a summary on naming organic compounds, infographic 20.3b looks at the rules for decoding the types of organic compounds and how to name them.
Links to Enhanced Learning
For a general introduction to organic chemistry naming beyond the basic alkane naming, watch The Basics of Organic Nomenclature: Crash Course Organic Chemistry #2 – YouTube.
For interactive practice questions on isomers link to Organic Chemistry Practice from eCampusOntario H5P Studio.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from the following sources
- “12.3: The Structure of Organic Molecules – Alkanes and Their Isomers” & “12.6: Naming Alkanes” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.) by LibreTexts, licensed under CC BY-NC-SA 3.0 . / A derivative of 12.5: IUPAC Nomenclature, 12.2: Structures and Names of Alkanes, & 12.3: Branched-Chain Alkanes In Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
- “18.1 Hydrocarbons” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “12.2: Structures and Names of Alkanes” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: General, Organic, and Biological (V. 1.0), CC BY-NC-SA 3.0.
- 4-propyloctane ↵
-
- 3-methylpentane
- 2, 2-dimethylpentane
- 2-ethyl 2-methyloctane
↵
-
- See the image: 4-Ethyloctane | C10H22 | CID 85925 - PubChem (nih.gov)
- See the image: 3-Ethyl-2-methylpentane | C8H18 | CID 11863 - PubChem (nih.gov)
- See the image: 3,3,5-Trimethylheptane | C10H22 | CID 23544 - PubChem (nih.gov) ↵
Compounds with the same molecular formula but different spatial arrangements of the atoms in their molecules