Chapter 28 – Review
28.1 Carbohydrates
- Which compounds would be classified as carbohydrates? Check answer[1]
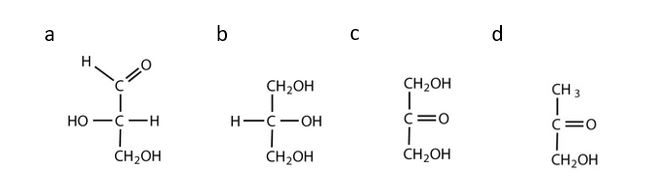
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
- Which compounds would be classified as carbohydrates? Check answer[2]
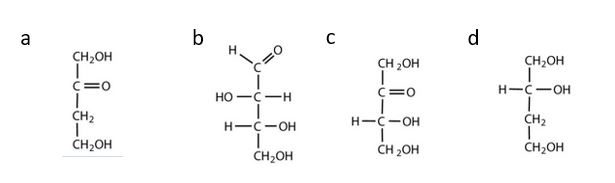
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
28.2 Lipids
- The melting point of elaidic acid is 52°C.
- What trend is observed when comparing the melting points of elaidic acid, oleic acid, and stearic acid? Explain.Check answer[3]
- Would you expect the melting point of palmitelaidic acid to be lower or higher than that of elaidic acid? Explain. Check answer[4]
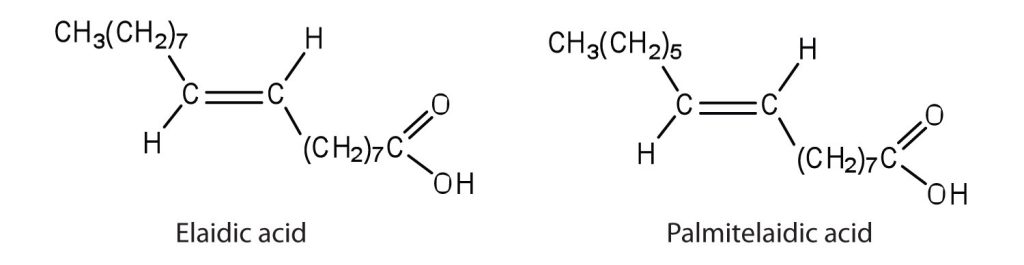
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
-
Examine the labels on two brands of margarine and two brands of shortening and list the oils used in the various brands.
- In cerebrosides (Figure 28.2s), is the linkage between the fatty acid and sphingosine an amide bond or an ester bond? Justify your answer.
- Explain whether each compound would be expected to diffuse through the lipid bilayer of a cell membrane.
- potassium chloride
- CH3CH2CH2CH2CH2CH3
- fructose
- Identify the role of each steroid hormone in the body.
- What fatty acid is the precursor for the prostaglandins? Check answer[9]
- Identify three biological effects of prostaglandins. Check answer[10]
- Why is it important to determine the ratio of LDLs to HDLs, rather than just the concentration of serum cholesterol?
28.3 Amino Acids, Proteins, and Enzymes
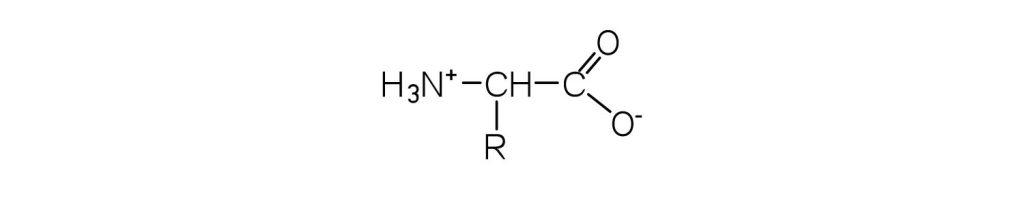
- What is the general structure of an α-amino acid? Check answer[11]
- Identify the amino acid that fits each description.
- Write the side chain of each amino acid.
- Write the side chain of each amino acid.
- Identify an amino acid whose side chain contains a(n)
- amide functional group.
- aromatic ring.
- carboxyl group.
- Identify an amino acid whose side chain contains a(n)
- OH group
- branched chain
- amino group
- Define each term.
- Draw the structure for the anion formed when alanine (at neutral pH) reacts with a base. Check answer[23]
- Draw the structure for the cation formed when alanine (at neutral pH) reacts with an acid. Check answer[24]
- Distinguish between the N-terminal amino acid and the C-terminal amino acid of a peptide or protein. Check answer[25]
- Describe the difference between an amino acid and a peptide. Check answer[26]
- Amino acid units in a protein are connected by peptide bonds. What is another name for the functional group linking the amino acids? Check answer[27]
- Draw the structure for each peptide.
- Identify the C- and N-terminal amino acids for the peptide lys-val-phe-gly-arg-cys. Check answer[30]
- Identify the C- and N-terminal amino acids for the peptide asp-arg-val-tyr-ile-his-pro-phe.
- What is the predominant attractive force that stabilizes the formation of secondary structure in proteins? Check answer[31]
- Distinguish between the tertiary and quaternary levels of protein structure. Check answer[32]
- Briefly describe four ways in which a protein could be denatured. Check answer[33]
- What name is given to the predominant secondary structure found in silk? Check answer[34]
- What name is given to the predominant secondary structure found in wool protein?
- A protein has a tertiary structure formed by interactions between the side chains of the following pairs of amino acids. For each pair, identify the strongest type of interaction between these amino acids.
- What level(s) of protein structure is(are) ordinarily disrupted in denaturation? What level(s) is(are) not? Check answer[39]
- Distinguish between the lock-and-key model and induced-fit model of enzyme action. Check answer[40]
28.4 Nucleic Acids and DNA
- Name the two kinds of nucleic acids. Check answer[41]
- Which type of nucleic acid stores genetic information in the cell? Check answer[42]
- What are complementary bases? Check answer[43]
- Why is it structurally important that a purine base always pair with a pyrimidine base in the DNA double helix? Check answer[44]
- For this short RNA segment,
- identify the 5′ end and the 3′ end of the molecule.
- circle the atoms that comprise the backbone of the nucleic acid chain.
- write the nucleotide sequence of this RNA segment.
Check answer[45]
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
- For this short DNA segment,
- identify the 5′ end and the 3′ end of the molecule.
- circle the atoms that comprise the backbone of the nucleic acid chain
- write the nucleotide sequence of this DNA segment.
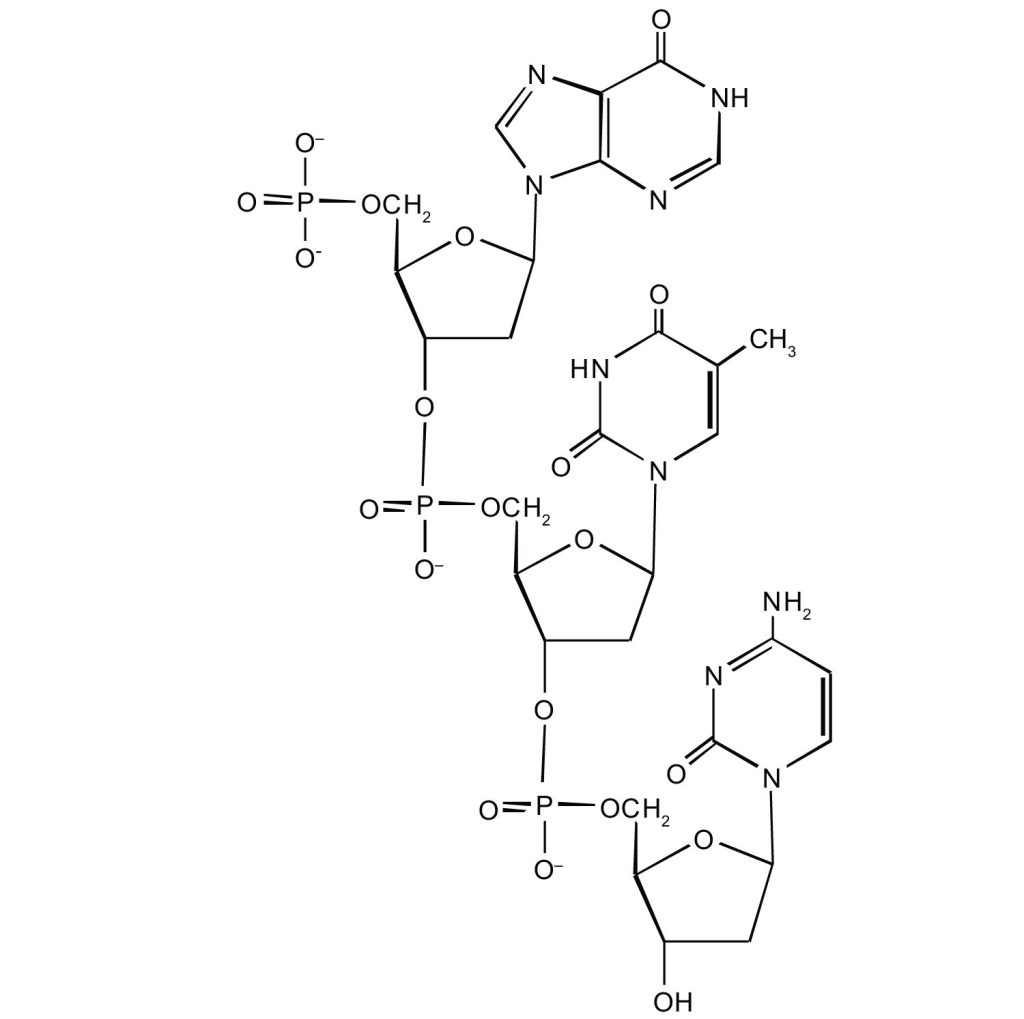
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
- Which nitrogenous base in DNA pairs with each nitrogenous base?
- Which nitrogenous base in RNA pairs with each nitrogenous base?
- cytosine
- adenine
- guanine
- thymine
- How many hydrogen bonds can form between the two strands in the short DNA segment shown below?
5′ ATGCGACTA 3′ 3′ TACGCTGAT 5′ Check answer[50] - How many hydrogen bonds can form between the two strands in the short DNA segment shown below?
5′ CGATGAGCC 3′ 3′ GCTACTCGG 5′ - Identify the three molecules needed to form the nucleotides in each nucleic acid.
- Classify each compound as a pentose sugar, a purine, or a pyrimidine.
- What is the sugar unit in each nucleic acid?
- For each structure, circle the sugar unit and identify the nucleotide as a ribonucleotide or a deoxyribonucleotide. Check answer[61]
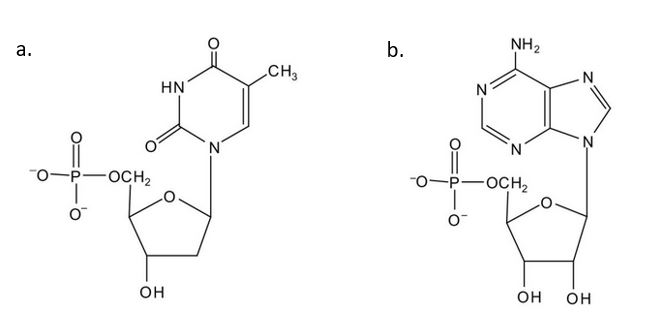
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0). - For each structure, circle the nitrogenous base and identify it as a purine or pyrimidine. Check answer[62]
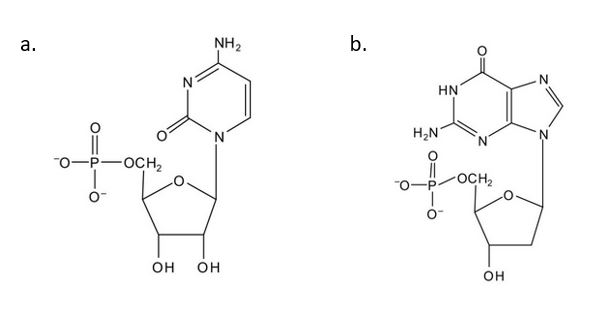
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0). - In DNA replication, a parent DNA molecule produces two daughter molecules. What is the fate of each strand of the parent DNA double helix? Check answer[63]
- What is the role of DNA in transcription? What is produced in transcription? Check answer[64]
- Describe how replication and transcription are similar. Check answer[65]
- Describe how replication and transcription differ.
- A portion of the coding strand for a given gene has the sequence 5′‑ATGAGCGACTTTGCGGGATTA‑3′.
- A portion of the coding strand for a given gene has the sequence 5′‑ATGGCAATCCTCAAACGCTGT‑3′.
- What is the sequence of complementary template strand?
- What is the sequence of the mRNA that would be produced during transcription from this segment of DNA?
- What are the roles of mRNA and tRNA in protein synthesis? Check answer[68]
- What is the initiation codon? Check answer[69]
- What are the termination codons and how are they recognized? Check answer[70]
- Write the anticodon on tRNA that would pair with each mRNA codon.
- Write the codon on mRNA that would pair with each tRNA anticodon.
- 5′‑UUG‑3′
- 5′‑GAA‑3′
- 5′‑UCC‑3′
- 5′‑CAC‑3′
- The peptide hormone oxytocin contains 9 amino acid units. What is the minimum number of nucleotides needed to code for this peptide? Check answer[75]
- Myoglobin, a protein that stores oxygen in muscle cells, has been purified from a number of organisms. The protein from a sperm whale is composed of 153 amino acid units. What is the minimum number of nucleotides that must be present in the mRNA that codes for this protein?
- Use Figure 28.4r to determine the amino acid sequence produced from this mRNA sequence: 5′‑AUGAGCGACUUUGCGGGAUUA‑3′. Check answer[76]
- Use Figure 28.4r to determine the amino acid sequence produced from this mRNA sequence: 5′‑AUGGCAAUCCUCAAACGCUGU‑3′
- What effect can UV radiation have on DNA? Check answer[77]
- What causes PKU? Check answer[78]
- How is PKU detected and treated? Check answer[79]
- A portion of the coding strand of a gene was found to have the sequence 5′‑ATGAGCGACTTTCGCCCATTA‑3′. A mutation occurred in the gene, making the sequence 5′‑ATGAGCGACCTTCGCCCATTA‑3′.
- A portion of the coding strand of a gene was found to have the sequence 5′‑ATGGCAATCCTCAAACGCTGT‑3′. A mutation occurred in the gene, making the sequence 5′‑ATGGCAATCCTCAACGCTGT‑3′.
- Identify the mutation as a substitution, an insertion, or a deletion.
- What effect would the mutation have on the amino acid sequence of the protein obtained from this mutated gene?
- What is a mutagen? Check answer[82]
- Give two examples of mutagens. Check answer[83]
28.5 Vitamins
Attribution & References
Except where otherwise noted, this page (including images in solutions) is adapted by Gregory A. Anderson and Samantha Sullivan Sauer from
- “17.E: Exercises“, “18.E: Amino Acids, Proteins, and Enzymes (Exercises)” & “19.E: Nucleic Acids” (Exercises) In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- “18.1 Properties of Amino Acids” & “18.3 Peptides” In Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
- Images in solutions are from the original source, except:
- 28.3 Question 1, 3, 4: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0
- 28.3 Question 8, 9: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0
- 28.3 Question 13: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0
- 28.4 Question 5: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0
- 28.5 Question 14, 15: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0
- a. This is a carbohydrate because the molecule contains an aldehyde functional group with OH groups on the other two carbon atoms. b. This is not a carbohydrate because the molecule does not contain an aldehyde or a ketone functional group. c. This is a carbohydrate because the molecule contains a ketone functional group with OH groups on the other two carbon atoms. d. This is not a carbohydrate; although it has a ketone functional group, one of the other carbons atoms does not have an OH group attached. ↵
- a. This is not a carbohydrate; although it has a ketone functional group, one of the other carbons atoms does not have an OH group attached. b. This is a carbohydrate because the molecule contains an aldehyde functional group with OH groups on the other two carbon atoms. c. This is a carbohydrate because the molecule contains a ketone functional group with OH groups on the other two carbon atoms. d. This is not a carbohydrate; it does not contain an aldehyde group, nor does it contain a ketone group. ↵
- Stearic acid has the highest melting point, followed by elaidic acid, and then oleic acid with the lowest melting point. Elaidic acid is a trans fatty acid, and the carbon chains can pack together almost as tightly as those of the saturated stearic acid. Oleic acid is a cis fatty acid, and the bend in the hydrocarbon chain keeps these carbon chains from packing as closely together; fewer interactions lead to a much lower melting point. ↵
- The melting point of palmitelaidic acid should be lower than that of elaidic acid because it has a shorter carbon chain (16, as compared to 18 for elaidic acid). The shorter the carbon chain, the lower the melting point due to a decrease in intermolecular interactions. ↵
- regulates the menstrual cycle and maintains pregnancy ↵
- regulates salt metabolism by stimulating the kidneys to retain sodium and excrete potassium ↵
- stimulates and maintains male sex characteristics ↵
- stimulates the conversion of proteins to carbohydrates ↵
- arachidonic acid ↵
- induce smooth muscle contraction, lower blood pressure, and contribute to the inflammatory response ↵
 ↵
↵- aspartic acid ↵
- arginine ↵
- glycine ↵
- CH2OH− ↵
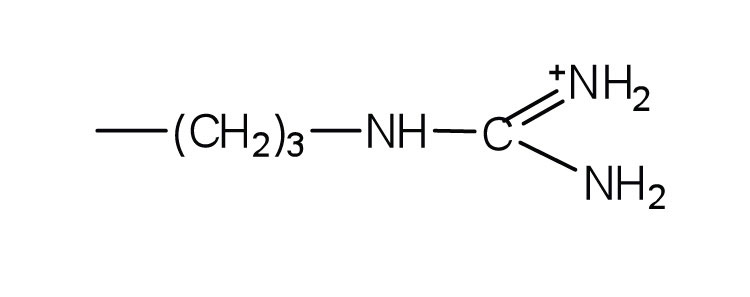 ↵
↵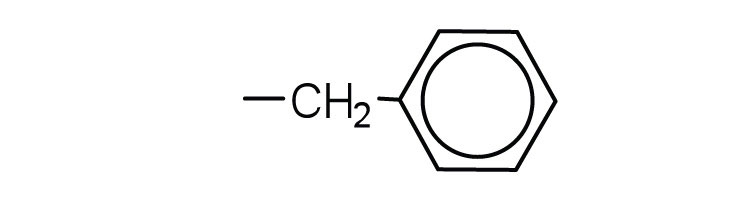 ↵
↵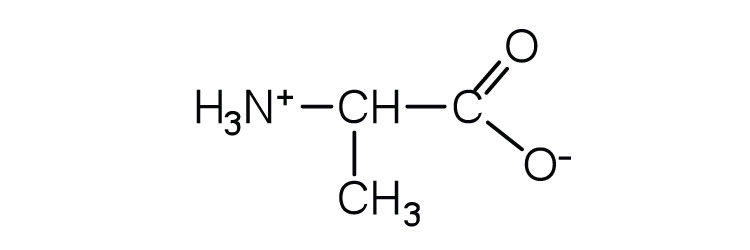 ↵
↵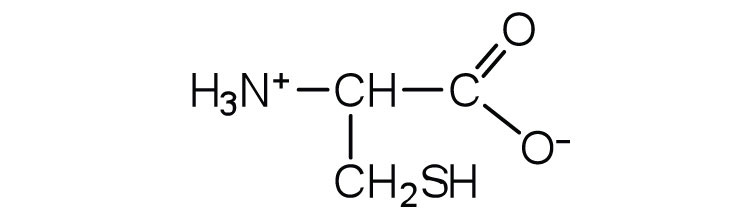 ↵
↵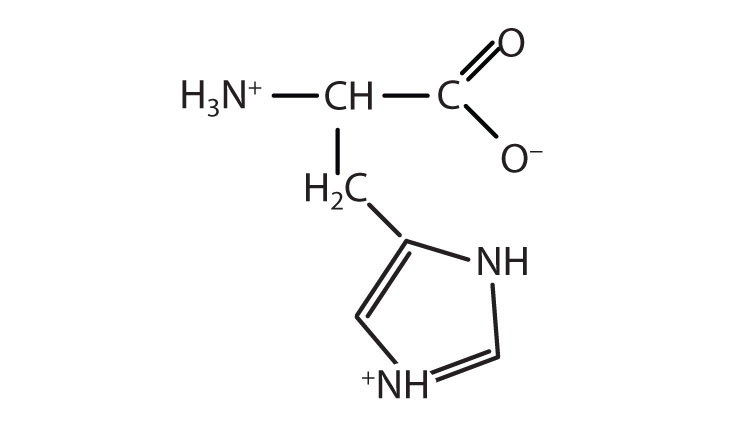 ↵
↵- an electrically neutral compound that contains both negatively and positively charged groups ↵
- the pH at which a given amino acid exists in solution as a zwitterion ↵
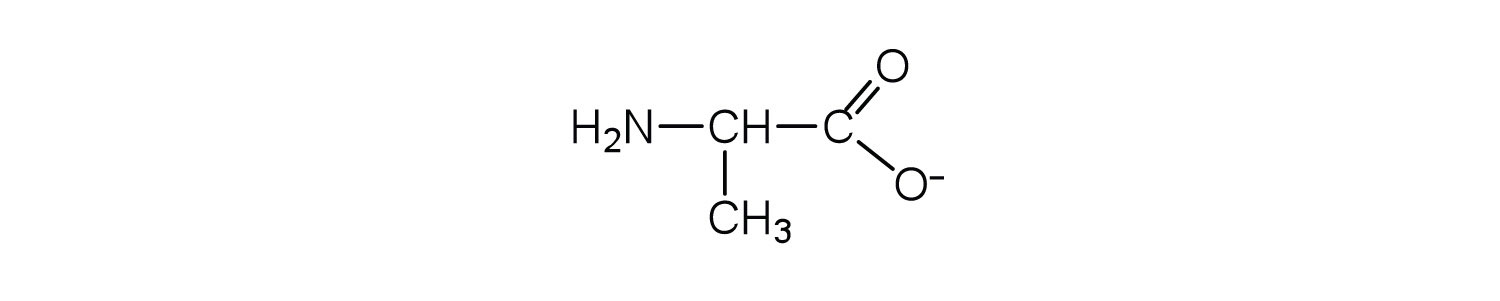 ↵
↵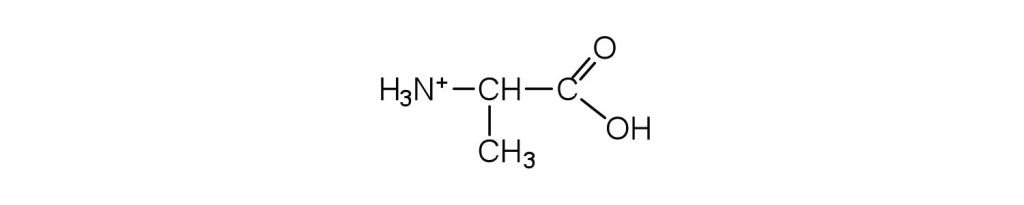 ↵
↵- The N-terminal end is the end of a peptide or protein whose amino group is free (not involved in the formation of a peptide bond), while the C-terminal end has a free carboxyl group. ↵
- A peptide is composed of two or more amino acids. Amino acids are the building blocks of peptides. ↵
- amide bond ↵
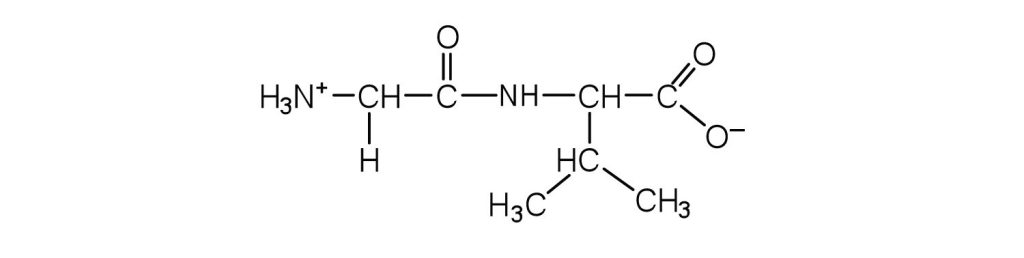 ↵
↵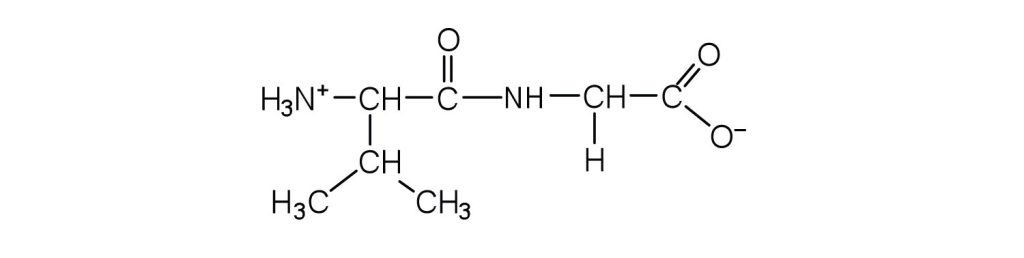 ↵
↵- C-terminal amino acid: cys; N-terminal amino acid: lys ↵
- hydrogen bonding ↵
- Tertiary structure refers to the unique three-dimensional shape of a single polypeptide chain, while quaternary structure describes the interaction between multiple polypeptide chains for proteins that have more than one polypeptide chain. ↵
- (1) heat a protein above 50°C or expose it to UV radiation; (2) add organic solvents, such as ethyl alcohol, to a protein solution; (3) add salts of heavy metal ions, such as mercury, silver, or lead; and (4) add alkaloid reagents such as tannic acid ↵
- β-pleated sheet ↵
- ionic bonding ↵
- dispersion forces ↵
- dispersion forces ↵
- disulfide linkage ↵
- Protein denaturation disrupts the secondary, tertiary, and quaternary levels of structure. Only primary structure is unaffected by denaturation. ↵
- The lock-and-key model portrays an enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site. The induced fit model portrays the enzyme structure as more flexible and is complementary to the substrate only after the substrate is bound. ↵
- deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) ↵
- DNA ↵
- the specific base pairings in the DNA double helix in which guanine is paired with cytosine and adenine is paired with thymine ↵
- The width of the DNA double helix is kept at a constant width, rather than narrowing (if two pyrimidines were across from each other) or widening (if two purines were across from each other). ↵
- ACU
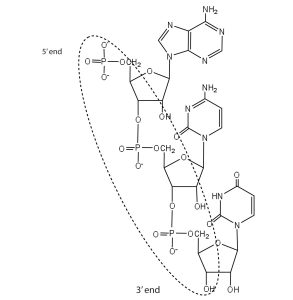 ↵
↵ - guanine ↵
- thymine ↵
- cytosine ↵
- adenine ↵
- 22 (2 between each AT base pair and 3 between each GC base pair) ↵
- nitrogenous base (adenine, guanine, cytosine, and thymine), 2-deoxyribose, and H3PO4 ↵
- nitrogenous base (adenine, guanine, cytosine, and uracil), ribose, and H3PO4 ↵
- purine ↵
- purine ↵
- pentose sugar ↵
- pyrimidine ↵
- pentose sugar ↵
- pyrimidine ↵
- ribose ↵
- deoxyribose ↵
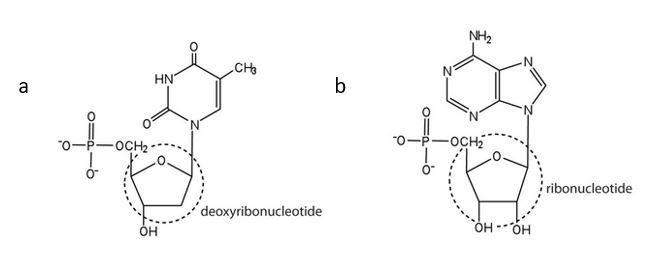 ↵
↵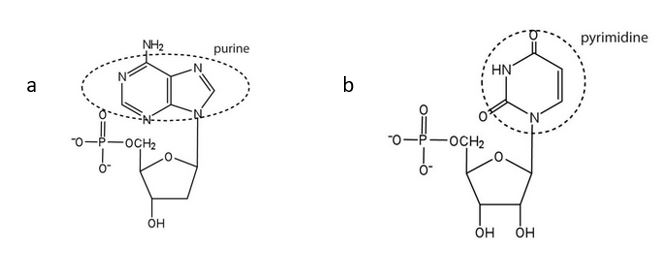 ↵
↵- Each strand of the parent DNA double helix remains associated with the newly synthesized DNA strand. ↵
- DNA serves as a template for the synthesis of an RNA strand (the product of transcription). ↵
- Both processes require a template from which a complementary strand is synthesized. ↵
- 3′‑TACTCGCTGAAACGCCCTAAT‑5′ ↵
- 5′‑AUGAGCGACUUUGCGGGAUUA‑3′ ↵
- mRNA provides the code that determines the order of amino acids in the protein; tRNA transports the amino acids to the ribosome to incorporate into the growing protein chain. ↵
- AUG ↵
- UAA, UAG, and UGA; they are recognized by special proteins called release factors, which signal the end of the translation process. ↵
- 3′‑AAA‑5′ ↵
- 3′‑GUA‑5′ ↵
- 3′‑UCG‑5′ ↵
- 3′‑GGC‑5′ ↵
- 27 nucleotides (3 nucleotides/codon) ↵
- met-ser-asp-phe-ala-gly-leu ↵
- It can lead to the formation of a covalent bond between two adjacent thymines on a DNA strand, producing a thymine dimer. ↵
- the absence of the enzyme phenylalanine hydroxylase ↵
- PKU is diagnosed by assaying a sample of blood or urine for phenylalanine or one of its metabolites; treatment calls for an individual to be placed on a diet containing little or no phenylalanine. ↵
- substitution ↵
- Phenylalanine (UUU) would be replaced with leucine (CUU). ↵
- a chemical or physical agent that can cause a mutation ↵
- UV radiation and gamma radiation (answers will vary) ↵
- a) fat soluble, b) water soluble, c) water soluble ↵
- a) needed for the formation of vision pigments, b) needed for cell growth and metabolism c) needed for blood clotting ↵

