26.5 Amides – Structures, Properties and Naming
Learning Objectives
By the end of this section, you will be able to:
- Identify the general structure for an amide.
- Identify the functional group for an amide.
- Names amides with common names.
- Name amides according to the IUPAC system.
- Compare the boiling points of amides with alcohols of similar molar mass.
- Compare the solubilities in water of amides of five or fewer carbon atoms with the solubilities of comparable alkanes and alcohols in water.
Amides are molecules that contain nitrogen atoms connected to the carbon atom of a carbonyl group. If the two remaining bonds on the nitrogen atom are attached to hydrogen atoms, the compound is a simple amide. If one or both of the two remaining bonds on the atom are attached to alkyl or aryl groups, the compound is a substituted amide (Figure 26.5a.).
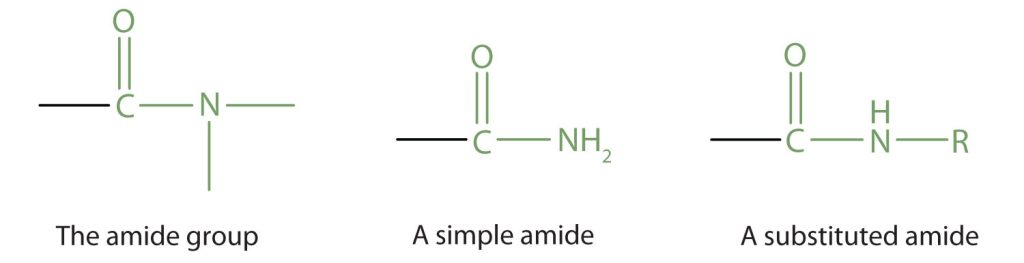
The carbonyl carbon-to-nitrogen bond is called an amide linkage. This bond is quite stable and is found in the repeating units of protein molecules, where it is called a peptide linkage.
Naming Amides
- Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide.
- The carbonyl carbon is numbered carbon 1 on it’s location. It is not necessary to include the location number in the name because it is assumed that the functional group will be on the end of the parent chain.
- Secondary amides are named by using an upper case N to designate that the alkyl group is on the nitrogen atom. Alkyl groups attached to the nitrogen are named as substituents. The letter N is used to indicate they are attached to the nitrogen.
- Tertiary amides are named in the same way as secondary amides, but with two N’s
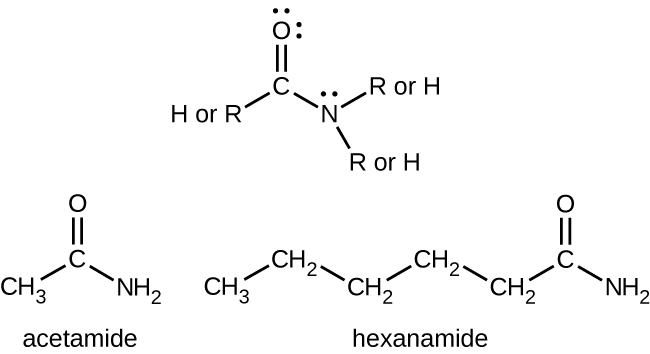
Simple amides are named as derivatives of carboxylic acids. The –ic ending of the common name or the –oic ending of the International Union of Pure and Applied Chemistry (IUPAC) name of the carboxylic acid is replaced with the suffix –amide (Figure 26.5b.).

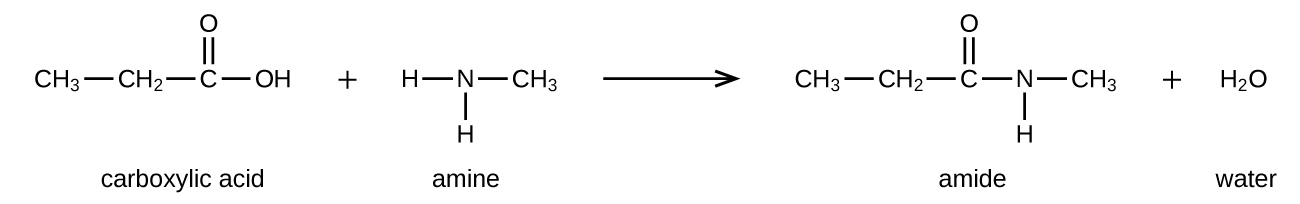
Amides can be produced when carboxylic acids react with amines or ammonia in a process called amidation. A water molecule is eliminated from the reaction, and the amide is formed from the remaining pieces of the carboxylic acid and the amine (Figure 26.5d.) (note the similarity to formation of an ester from a carboxylic acid and an alcohol discussed in the previous section).
Example 26.5a
Give the IUPAC name for the following amides.
- CH3CH2C=O(NH2)
- CH3C=O(NH)CH2CH3
Solution
- propanamide (proprionamide)
- N-ethylethanamide (N-ethylacetamide)
Exercise 26.5a
Write the IUPAC name for each of the following amides.
- CH3CH2CH2CH2C=O(NH2)
- CH3CH2CHClCH2C=O(NH2)
Check your answers: [1]
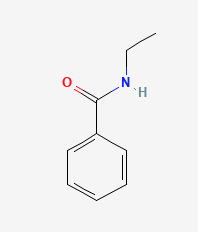
Exercise 26.5b
Name each compound with the common name, the IUPAC name or both.
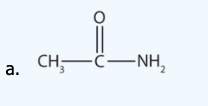
b.
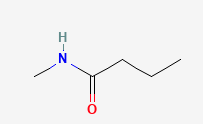
Check your answers: [2]
The reaction between amines and carboxylic acids to form amides is biologically important. It is through this reaction that amino acids (molecules containing both amine and carboxylic acid substituents) link together in a polymer to form proteins.
Proteins and Enzymes
Proteins are large biological molecules made up of long chains of smaller molecules called amino acids. Organisms rely on proteins for a variety of functions—proteins transport molecules across cell membranes, replicate DNA, and catalyze metabolic reactions, to name only a few of their functions. The properties of proteins are functions of the combination of amino acids that compose them and can vary greatly. Interactions between amino acid sequences in the chains of proteins result in the folding of the chain into specific, three-dimensional structures that determine the protein’s activity.
Enzymes are large biological molecules, mostly composed of proteins, which are responsible for the thousands of metabolic processes that occur in living organisms. Enzymes are highly specific catalysts; they speed up the rates of certain reactions. Enzymes function by lowering the activation energy of the reaction they are catalyzing, which can dramatically increase the rate of the reaction. Most reactions catalyzed by enzymes have rates that are millions of times faster than the non-catalyzed version.
For more information on proteins see Chapter 28.3 Amino Acids, Proteins and Enzymes.
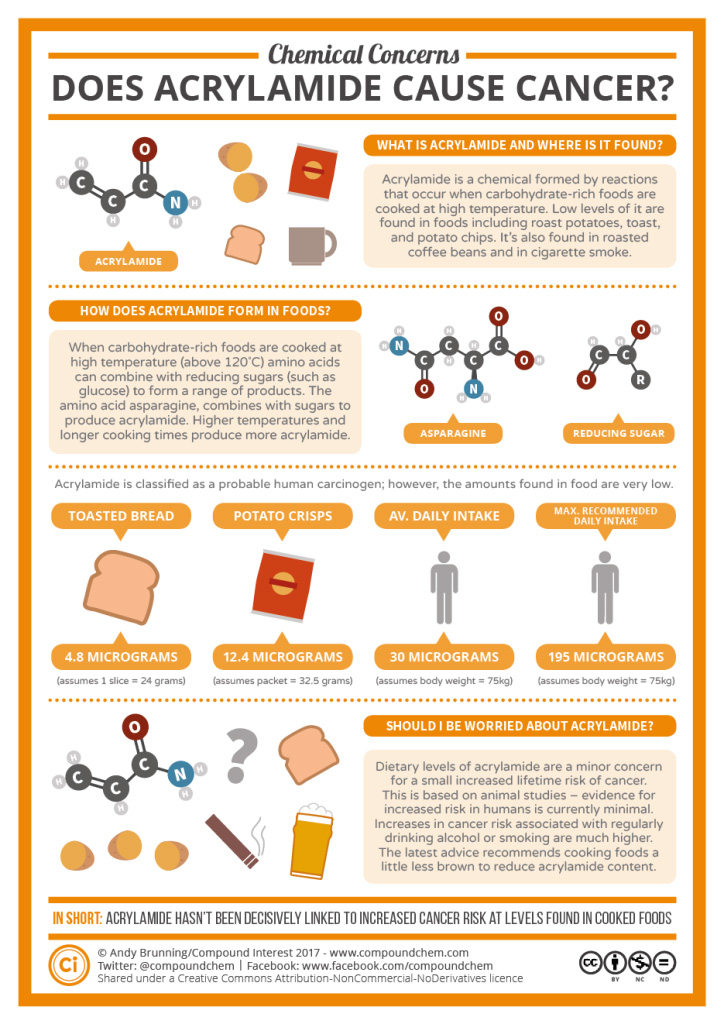
Spotlight on Everyday Chemistry: Acrylamide
Acrylamide is found mainly in foods made from plants, that are high in carbohydrates and low in proteins. Examples of such foods include potato products, grain products or coffee. Acrylamide is more likely to build up when foods are cooked for longer periods of time or at higher temperatures. Acrylamide is known to cause cancer in experimental animals and is therefore a possible human carcinogen.
Watch Properties of Amides on YouTube (8 min).
Video Source: Professor Dave Explains. (2019, September 19). Properties of Amides. [Video]. YouTube.
Indigenous Perspectives: Hakarl (Iceland)
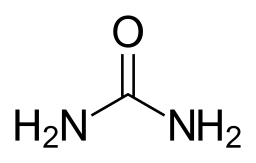
Hakarl is a fermented Greenland shark, which is the national dish of Iceland. The process of fermenting the shark is essential as fresh shark meat is poisonous as it contains urea (Figure 26.5e.) and trimethylamine oxide. See the Day 13: Iceland: Hákarl infographic for further information.
Physical Properties of Amides
With the exception of formamide (HCONH2), which is a liquid, all simple amides are solids (Table 26.5a.). The lower members of the series are soluble in water, with borderline solubility occurring in those that have five or six carbon atoms. Like the esters, solutions of amides in water usually are neutral—neither acidic nor basic.
| Condensed Structural Formula | Name | Melting Point (°C) | Boiling Point (°C) | Solubility in Water |
|---|---|---|---|---|
| HCONH2 | formamide | 2 | 193 | soluble |
| CH3CONH2 | acetamide | 82 | 222 | soluble |
| CH3CH2CONH2 | propionamide | 81 | 213 | soluble |
| CH3CH2CH2CONH2 | butyramide | 115 | 216 | soluble |
| C6H5CONH2 | benzamide | 132 | 290 | slightly soluble |
Source: “15.14: Physical Properties of Amides” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
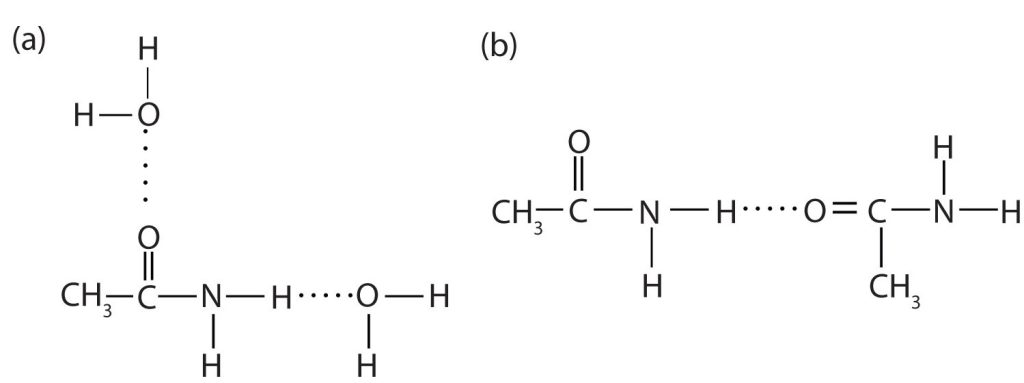
The amides generally have high boiling points and melting points. These characteristics and their solubility in water result from the polar nature of the amide group and hydrogen bonding (Figure 26.5f.). (Similar hydrogen bonding plays a critical role in determining the structure and properties of proteins, deoxyribonucleic acid [DNA], ribonucleic acid [RNA], and other giant molecules so important to life processes.
Spotlight on Everyday Chemistry: Urea
Urea (CO(NH2)2) is a diamide containing two amide groups joined by a carbonyl functional group. Urea is the main component of urine consisting of nitrogenous waste products from the metabolic breakdown of proteins. The liver produces enzymes which form urea which is then transported to the kidney’s for removal from the body. Urea a colourless, odourless solid which is highly soluble in water and when dissolved in water is neither acidic nor alkaline. Urea is widely used in fertilizers as a nitrogen source and is an important raw material for chemical industry.

Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey from:
- “15.13: Amides- Structures and Names” & “15.14: Physical Properties of Amides” In Basics of General, Organic, and Biological Chemistry (Ball et al.)by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- “18.4 Amines and Amides” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
References cited in-text
National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 11959, N-Ethylbenzamide. Retrieved February 7, 2024.
National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 28774, N-Methylbutyramide. Retrieved February 7, 2024
organic molecule that features a nitrogen atom connected to the carbon atom in a carbonyl group
the reaction in which an amide group replaces the hydrogen atom on the amino group.

