24.1 The Carbonyl Group
Learning Objectives
By the end of this section, you will be able to:
- Identify the aldehyde and ketone functional group
- Identify the general structure for an aldehyde and a ketone
Organic molecules that contain a carbon atom connected to an oxygen atom by a double bond make up several important groups of molecules. This functional group, called the carbonyl group, contains a trigonal planar carbon that can attach to two other substituents leading to several subfamilies. In this chapter we will consider the aldehydes and ketones, and in the next chapter we will consider the carboxylic acids and esters, all of which contain this carbonyl group.
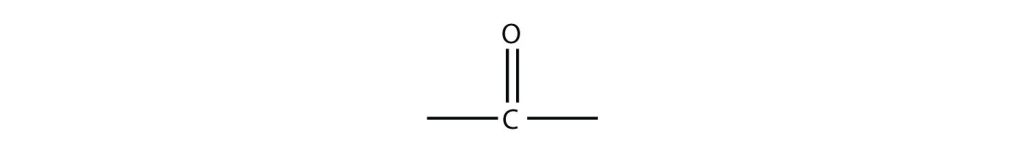
As mentioned, the carbonyl group has a carbon-to-oxygen double bond, as seen in figure 24.1a.
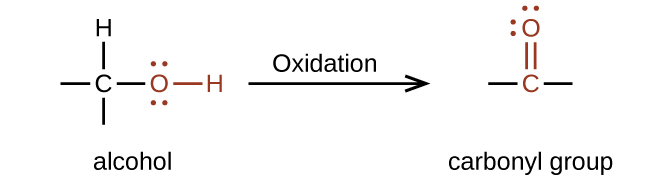
Typically, carbonyl groups are formed by the oxidation of an alcohol, as shown in figure 24.1b.
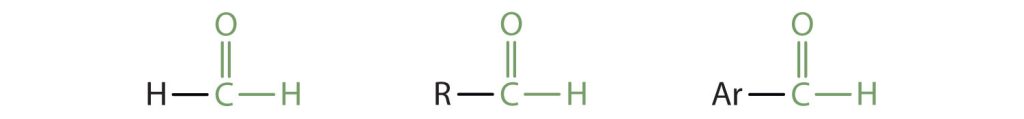
Carbonyl groups that are attached to hydrogen atoms or other groups of carbons define two related families of organic compounds: the aldehydes and the ketones. The carbonyl group is ubiquitous in biological compounds. It is found in carbohydrates, fats, proteins, nucleic acids, hormones, and vitamins—organic compounds critical to living systems.
In an aldehyde, at least one of the attached groups must be a hydrogen atom. The compounds shown in Figure 24.1c. are aldehydes.
In a ketone, two carbon groups are attached to the carbonyl carbon atom. The general formulas shown in Figure 24.1d. depict several ketones, in which R represents an alkyl group and Ar stands for an aryl (aromatic) group.
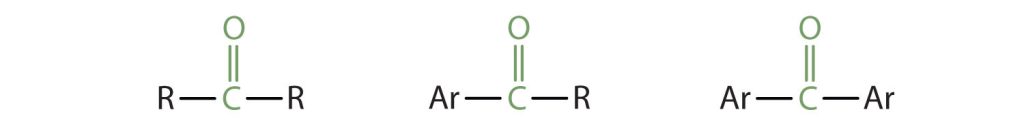
In condensed formulas, we use CHO to identify an aldehyde rather than COH, which might be confused with an alcohol. This follows the general rule that in condensed structural formulas H comes after the atom it is attached to (usually C, N, or O), as shown in Figure 24.1e.

The carbon-to-oxygen double bond is not shown but understood to be present in condensed formulas. Because they contain the same functional group, aldehydes and ketones share many common properties, but they still differ enough to warrant their classification into two families.
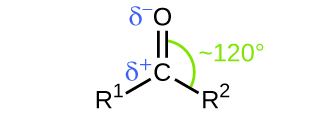
In both aldehydes and ketones, the geometry around the carbon atom in the carbonyl group is trigonal planar; the carbon atom exhibits sp2 hybridization. Two of the sp2 orbitals on the carbon atom in the carbonyl group are used to form σ bonds to the other carbon or hydrogen atoms in a molecule. The remaining sp2 hybrid orbital forms a σ bond to the oxygen atom. The unhybridized p orbital on the carbon atom in the carbonyl group overlaps a p orbital on the oxygen atom to form the π bond in the double bond.
Like the [latex]\text{C} = \text{O}[/latex] bond in carbon dioxide, the [latex]\text{C} = \text{O}[/latex] bond of a carbonyl group is polar (recall that oxygen is significantly more electronegative than carbon, and the shared electrons are pulled toward the oxygen atom and away from the carbon atom). Many of the reactions of aldehydes and ketones start with the reaction between a Lewis base and the carbon atom at the positive end of the polar [latex]\text{C} = \text{O}[/latex] bond to yield an unstable intermediate that subsequently undergoes one or more structural rearrangements to form the final product (Figure 24.1f.).
Though we will get into the nomenclature rules for aldehydes and ketones in the next part of this chapter, it warrants previewing it here. When naming aldehydes, the main chain of C atoms must include the carbon in the carbonyl group, which is numbered as position 1 in the carbon chain. The parent name of the hydrocarbon is used, but the suffix –al is appended. (Do not confuse –al with –ol, which is the suffix used for alcohols.) Figure 24.1g. shows the first three simplest aldheydes.
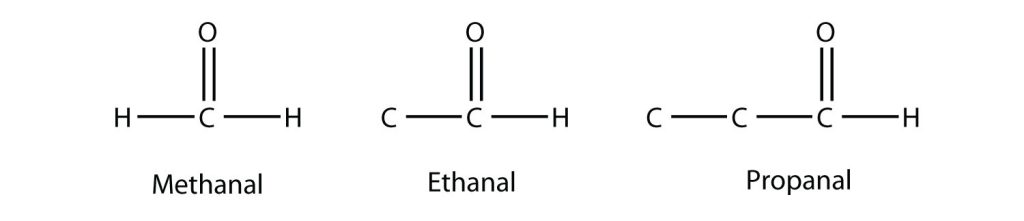
Methanal has a common name with which you may be familiar: formaldehyde. The main thing to note about aldehydes is that the carbonyl group is at the end of a carbon chain.
The smallest ketone has three C atoms in it. When naming a ketone, we take the name of the parent hydrocarbon and change the suffix to –one. Figure 24.1h. depicts the simplest ketone molecule.
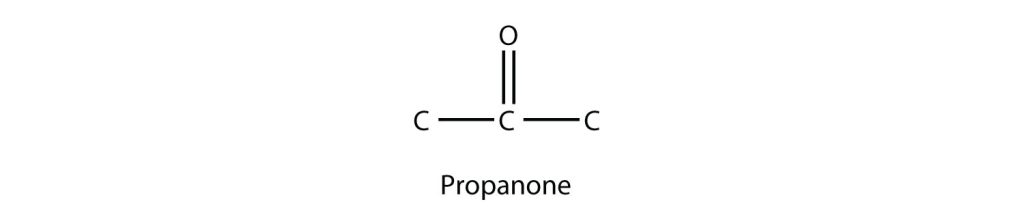
The common name for propanone is acetone. There is another way to name ketones: name the alkyl groups that are attached to the carbonyl group and add the word ketone to the name. So, propanone can also be called dimethyl ketone, while 2-butanone is called methyl ethyl ketone.
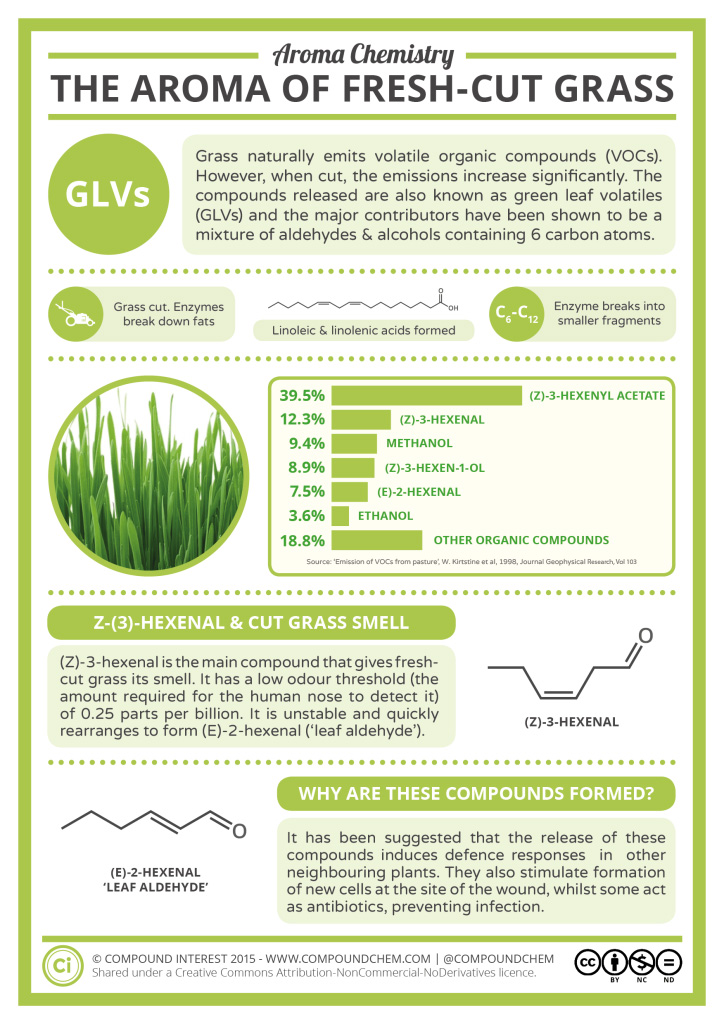
Spotlight on Everyday Chemistry: The Aroma of Fresh Cut Grass
Fresh cut grass has a recognizable scent that is based on an key aldehyde compound. Infographic 24.1a. highlights some of the chemistry behind fresh cut grass.
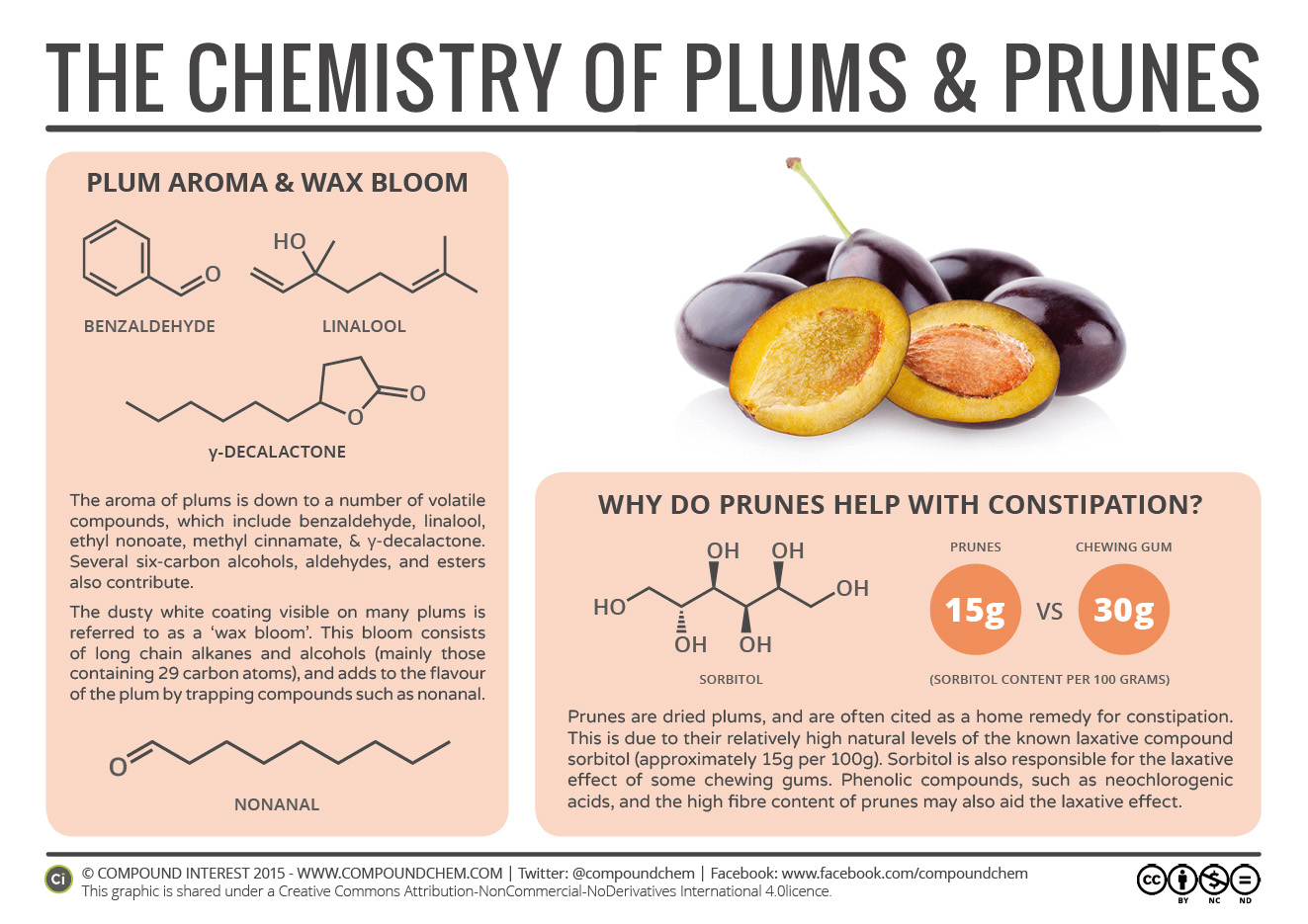
Spotlight on Everyday Chemistry: The Chemistry of Plums & Prunes – Constipation & Chewing Gum
The smell of plums is also based on aldehyde and ketone compounds. This means that prunes have aldehyde and ketone compounds too. Read more about plums and prunes in Infographic 24.1b.
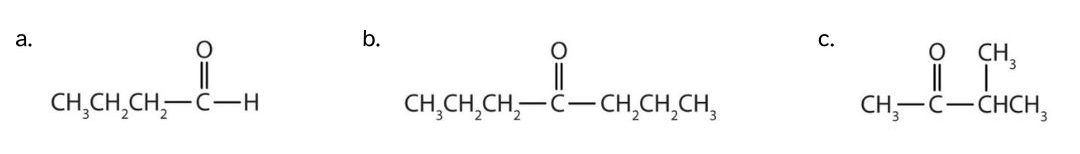
Exercise 24.1a
Classify each compound as an aldehyde or a ketone.
Solutions
- This compound has the carbonyl group on an end carbon atom, so it is an aldehyde.
- This compound has the carbonyl group on an interior carbon atom, so it is a ketone. Both alkyl groups are propyl groups.
- This compound has the carbonyl group between two alkyl groups, so it is a ketone. One alkyl group has three carbon atoms and is attached by the middle carbon atom; it is an isopropyl group. A group with one carbon atom is a methyl group.
Source: Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0.
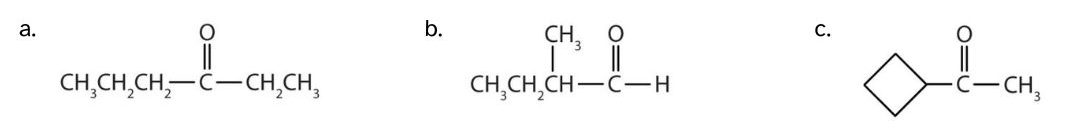
Classify each compound as an aldehyde or a ketone.
Check Your Answers:[1]
Source: Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0.
Attribution & References
Except where otherwise noted, this page is adapted by Gregory A. Anderson and Samantha Sullivan Sauer from
- “15.1: The Carbonyl Group” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Beginning Chemistry (Ball), CC BY-NC-SA 3.0, a LibreTexts version of Beginning Chemistry (v 1.0).
- “14.9: Aldehydes and Ketones- Structure and Names” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
-
- This compound has the carbonyl group on an interior carbon atom, so it is a ketone.
- This compound has the carbonyl group on an end carbon atom, so it is an aldehyde.
- This compound has the carbonyl group between two alkyl groups, so it is a ketone. One alkyl group has a four carbon ring of atoms and is thus a cyclobutyl group. The other alkyl group contains one carbon atom and is thus a methyl group.
↵
an group containing an aromatic ring

