19.5 Families of Organic Molecules – Functional Groups
Learning Objectives
By the end of this section, you will be able to:
- Identify and describe functional groups in organic molecules.
Organic molecules can be classified into families based on structural similarities. Within a family, molecules have similar physical behavior and often have predictable chemical reactivity. The structural components differentiating different organic families involve specific arrangements of atoms or bonds, called functional groups. If you understand the behavior of a particular functional group, you can describe the general properties of that class of compounds.
The simplest organic compounds are in the alkane family and contain only carbon–carbon and carbon–hydrogen single bonds but do not have any specific functional group. Hydrocarbons containing at least one carbon–carbon double bond, (denoted C=C), are in the alkene family. Alkynes have at least one carbon–carbon triple bond (C≡C). Both carbon–carbon double bonds and triple bonds chemically react in specific ways that differ from reactions of alkanes and each other, making these specific functional groups.
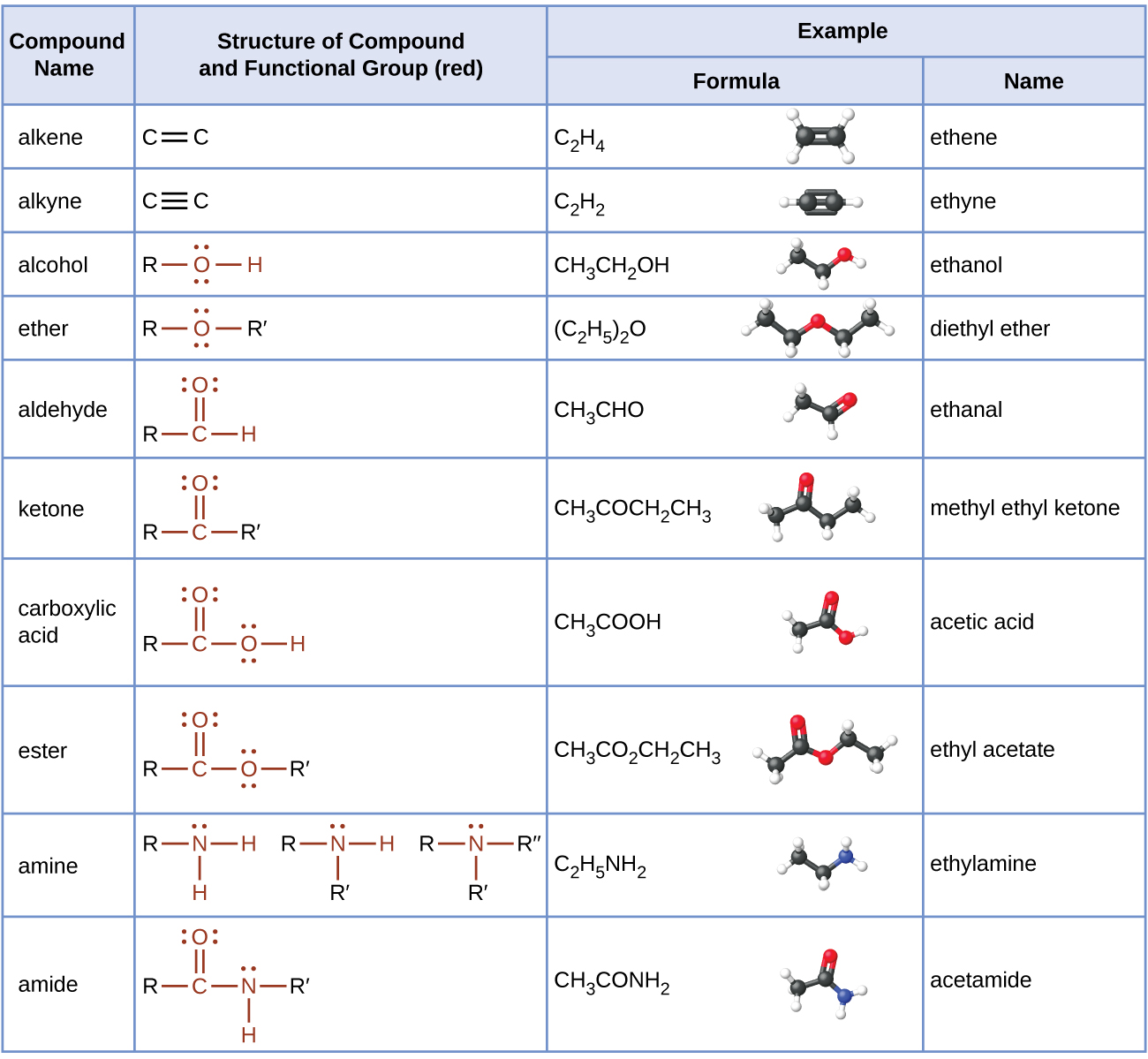
In the next few chapters, we will learn more about additional functional groups that are made up of atoms or groups of atoms attached to hydrocarbons. Being able to recognize different functional groups will help to understand and describe common medications and biomolecules such as amino acids, carbohydrates, and fats. Table 19.5a. below list several of the functional groups to become familiar with as you learn about organic chemistry.
The table here summarizes the structures discussed in this chapter:
Table 19.5a. Summary of the Classification of Organic Compounds.
Exercise 19.5a
Exercise 19.5a (text version)
Organic Functional Groups: Match the organic structure (1-10) with its functional group.
Structures:
- CH3CH3
- CH2=CH2
- CH≡CH
- CH3OH
- CH3COCH3
- CH3CHO
- CH3COOH
- CH3COOCH3
- CH3CONH2
- CH3NH2
Functional Group List:
amide, alkane, carboxylic acid, alkene, alcohol, ketone, alkyne, amine, aldehyde, ester
Check Your Answers:[1]
Source: Exercise 19.5a by Samantha Sullivan Sauer is licensed under CC BY-NC 4.0
Exercise 19.5b
Exercise 19.5b (text version)
Organic Functional Group Naming: Match the organic structure (1-10) with its functional group name.
Structures:
- CH3CH3
- CH2=CH2
- CH≡CH
- CH3OH
- CH3COCH3
- CH3CHO
- CH3COOH
- CH3COOCH3
- CH3CONH2
- CH3NH2
Functional Group Names List:
-amide, -ane, -oic acid, -ene, -ol, -one, -yne, -amine, -al, -oate
Check Your Answers:[2]
Source: Exercise 19.5b by Samantha Sullivan Sauer is licensed under CC BY-NC 4.0
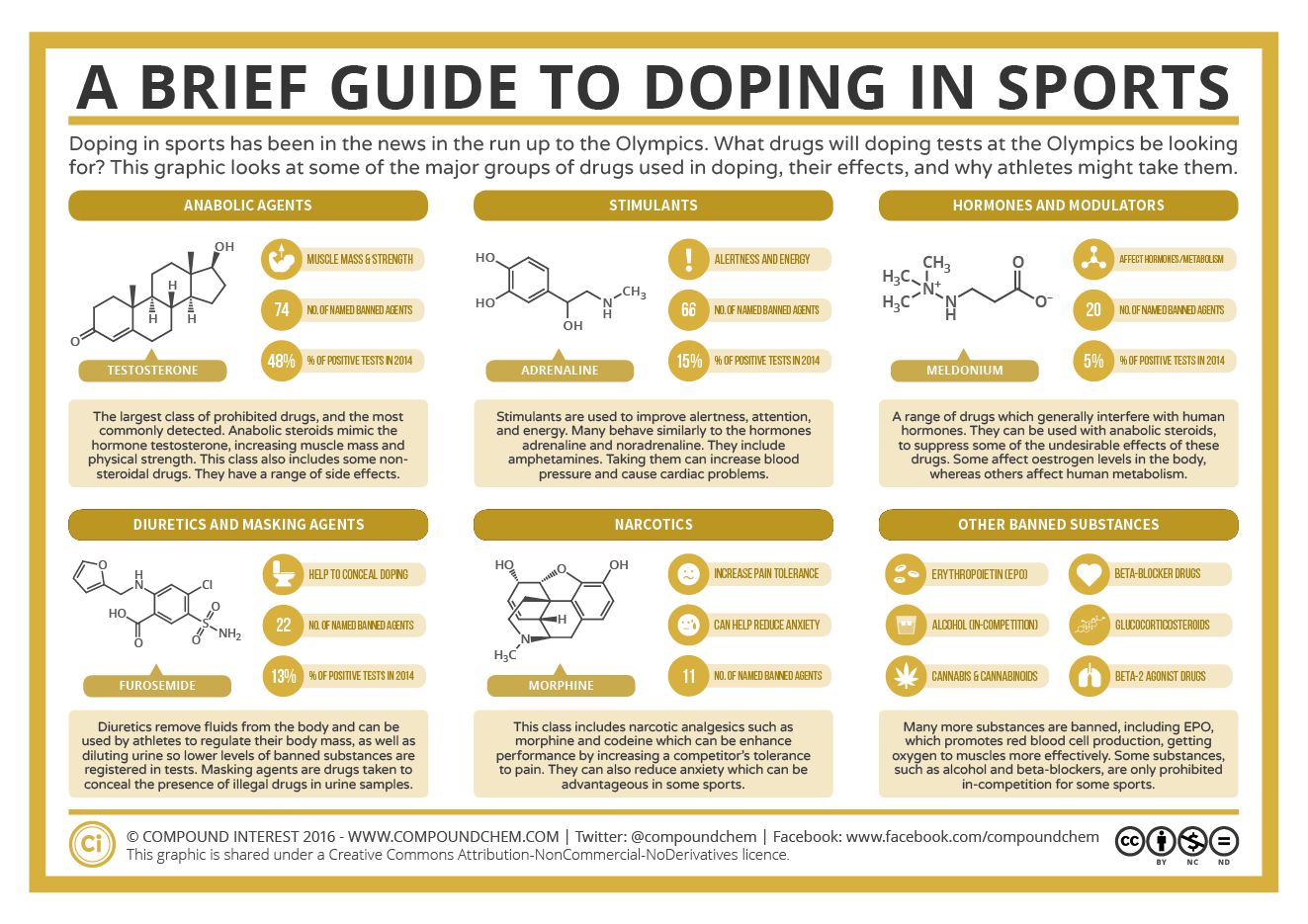
Spotlight on Everyday Chemistry: Doping in Sports
Doping in sports continues to make headlines as some athletes have turned to various drugs to enhance their performance against their opponents. Regulating bodies of professional sports monitor doping closely. The infographic looks at the major drugs used in doping.
Links to Enhanced Learning
Explore two infographics Functional Groups in Organic Chemistry (first infographic) and Functional Groups in Organic Chemistry (second infographic) by Compound Interest for an extensive summary of functional groups.
Complex molecules have multiple functional groups within them. Explore the infographics from Compound Interest for a comprehensive look at:
- Intravenous anesthetics drug molecules through A Brief Summary to Intravenous Anesthetics [New tab]
- General inhalant anesthetic drug molecules through A Brief Summary of Inhalation Anesthetics [New tab]
- Antidepressant drug molecules through Major Classes of Antidepressant Drugs [New tab]
- Common painkiller molecules such as aspirin, ibuprofen and tramadol through A Brief Guide to Selected Common Painkillers [New tab]
- Antibiotic molecules through Different Classes of Antibiotics – An Overview [New tab]
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from:
The structural components differentiating different organic families involve specific arrangements of atoms or bonds. This is the part of a molecule that imparts a specific chemical reactivity.

