Chapter 22 – Review
22.1 Alkenes and Alkynes – Structure and Naming
- Briefly identify the important distinctions between a saturated hydrocarbon and an unsaturated hydrocarbon. Check answer [1]
- Briefly identify the important distinctions between an alkene and an alkane.
- Classify each compound as saturated or unsaturated. Identify each as an alkane, an alkene, or an alkyne. Check answer[2]
-
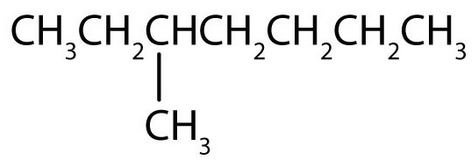
(credit: Intro Chem : GOB (v. 1.0), CC BY-NC 3.0) - CH3CH2C≡CCH3
-
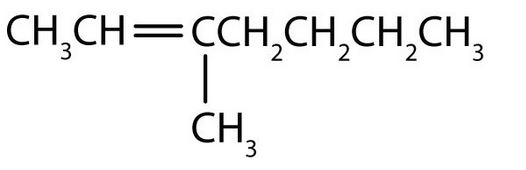
(credit: Intro Chem : GOB (v. 1.0), CC BY-NC 3.0)
-
- Briefly describe the physical properties of alkenes. How do these properties compare to those of the alkanes? Check answer[3]
- Without consulting tables, arrange the following alkenes in order of increasing boiling point: 1-butene, ethene, 1-hexene, and propene. Check answer[4]
- Without referring to a table or other reference, predict which member of each pair has the higher boiling point. Check answer[5]
1-pentene or 1-butene
3-heptene or 3-nonene - Which is a good solvent for cyclohexene, pentane or water?
- Draw the structure of each compound
- Name each compound. Check answer[9]
-
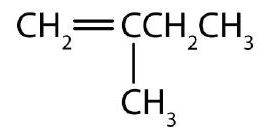
(credit: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0) -
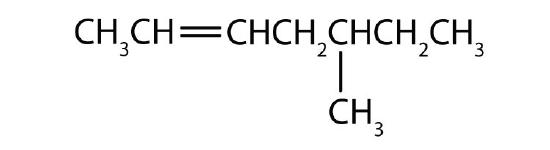
(credit: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0) -
(credit: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0)
-
- Briefly identify the important differences between an alkene and an alkyne. How are they similar? Check answer[10]
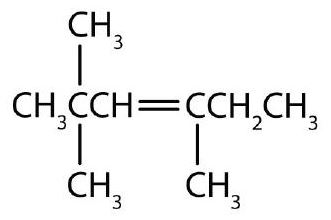
- The alkene (CH3)2CHCH2CH=CH2 is named 4-methyl-1-pentene. What is the name of (CH3)2CHCH2C≡CH? Check answer[11]
- Draw the structure for each compound.
- Name each alkyne. Check answer[14]
- CH3CH2CH2C≡CH
- CH3CH2CH2C≡CCH3
- What is wrong with each name? Draw the structure and give the correct name for each compound.
- 2-methyl-4-heptene
- 2-ethyl-2-hexene
- 2,2-dimethyl-3-pentene
22.2 Structure of Alkenes – Cis-Trans Isomers
- What are cis-trans (geometric) isomers? What two types of compounds can exhibit cis-trans isomerism? Check answer[15]
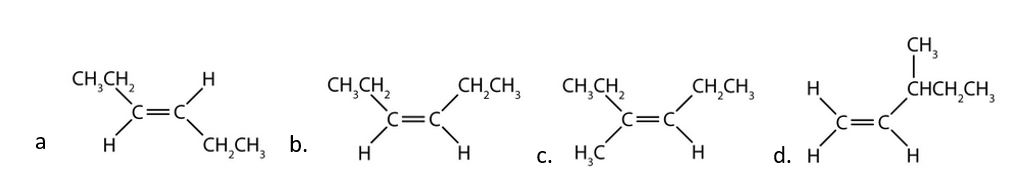
- Classify each compound as a cis isomer, a trans isomer, or neither. Check answer[16]
(credit: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0) - Do alkynes show cis-trans isomerism? Explain. Check answer[17]
- Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none.
- Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none.
- 2,3-dimethyl-2-pentene
- 1,1-dimethyl-2-ethylcyclopropane
- 1,2-dimethylcyclohexane
- 5-methyl-2-hexene
- 1,2,3-trimethylcyclopropane
22.3 Reactions of Alkenes and Alkynes
- What is the principal difference in properties between alkenes and alkanes? How are they alike? Check answer[23]
- If C12H24 reacts with HBr in an addition reaction, what is the molecular formula of the product? Check answer[24]
- Complete each equation.
- (CH3)2C=CH2 + Br2 → Check answer[25]
- CH2=C(CH3)CH2CH3+H2 → (with Ni) Check answer[26]
-
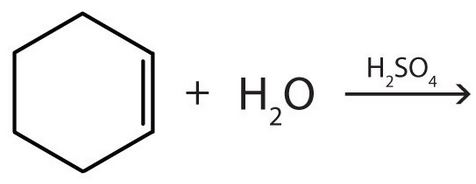
(credit: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0) Check answer[27]
- When three isomeric pentenes—X, Y, and Z—are hydrogenated, all three form 2-methylbutane. The addition of Cl2 to Y gives 1,2-dichloro-3-methylbutane, and the addition of Cl2 to Z gives 1,2-dichloro-2-methylbutane. Draw the original structures for X, Y, and Z.
- Describe Markovnikov’s Rule and give an example of water adding to an alkene that shows the application of this rule.
- Pentane and 1-pentene are both colourless, low-boiling liquids. Describe a simple test that distinguishes the two compounds. Indicate what you would observe. Check answer[28]
22.4 Aromatic Compounds – Structure and Naming
- Briefly identify the important characteristics of an aromatic compound. Check answer[29]
- Briefly describe the bonding in benzene. Check answer[30]
- What does the circle mean in the chemist’s representation of benzene? Check answer[31]
- What is meant by the prefixes meta, ortho, or para? Give the name and draw the structure for a compound that illustrates each. Check answer[32]
- What is a phenyl group? Give the structure for 3-phenyloctane. Check answer[33]
- Is each compound aromatic? Check answer[34]
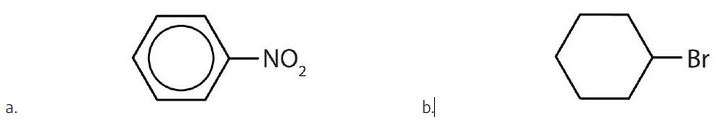
(credit: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0) - Is each compound aromatic? Check answer[35]
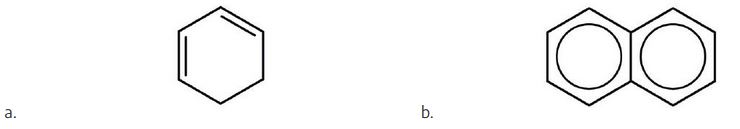
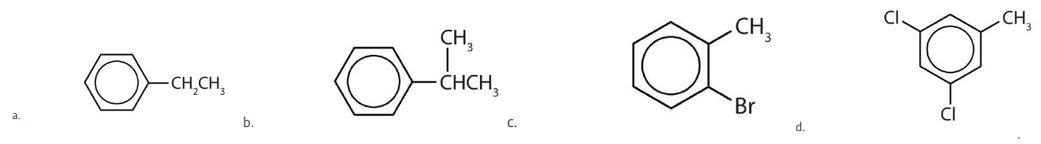
(credit: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0) - Draw the structure for each compound.
- Name each compound with its IUPAC name. Check answer[39]
(credit: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0) - What is wrong with each name? Check answer[40]
- 2-bromobenzene
- 3,3-dichlorotoluene
- 1,4-dimethylnitrobenzene
22.5 Aromatic Reactions
- How do the typical reactions of benzene differ from those of the alkenes? Check answer[41]
- Monobromination of toluene gives a mixture of three bromotoluene products. Draw and name them.
- How many products might be formed on chlorination of o-xylene (o-dimethylbenzene), m-xylene, and p-xylene?
- What is the major monosubstitution product from the Friedel–Crafts reaction of benzene with 1-chloro-2-methylpropane in the presence of AlCl3?
- What aromatic products would you obtain from the KMnO4 oxidation of the following substances?
-
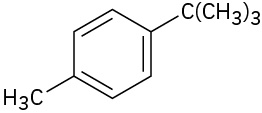
(credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0) -
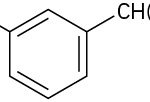
(credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0)
-
Links to Enhanced Learning
Create your own organic nomenclature quiz to identify, name and draw alkenes and alkynes using Organic Nomenclature (orgchem101.com). You can customize the types of questions you receive and get instant feedback.
Attribution & References
Except where otherwise noted, this page (including images in solutions) is adapted by David Wegman, Adrienne Richards and Samantha Sullivan Sauer from
- “ 13.E: Unsaturated and Aromatic Hydrocarbons (Exercises) “, in Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- “16.1 Electrophilic Aromatic Substitution Reactions: Bromination“, “16.2 Other Aromatic Substitutions“, “16.3 Alkylation and Acylation of Aromatic Rings: The Friedel–Crafts Reaction“, “16.8 Oxidation of Aromatic Compounds” and “16.9 Reduction of Aromatic Compounds” In Organic Chemistry (OpenStax) by John McMurray, CC BY-NC-SA 4.0. Access for free at Organic Chemistry (OpenStax)
- Images in solutions are from the original sources noted above, except:
- 22.1 Questions 8 and 12b: Intro Chem : GOB (v. 1.0), CC BY-NC 3.0
- 22.2 Question 4b, e: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0
- 22.3 Question 3c: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0
- 22.4 Questions 4, 5, 8: Intro Chem: GOB (v. 1.0),CC BY-NC 3.0
- Unsaturated hydrocarbons have double or triple bonds and are quite reactive; saturated hydrocarbons have only single bonds and are rather unreactive. ↵
- An alkene has a double bond; an alkane has single bonds only. a) saturated; alkane b) unsaturated; alkyne c) unsaturated; alkene ↵
- Alkenes have physical properties (low boiling points, insoluble in water) quite similar to those of their corresponding alkanes. ↵
- ethene < propene < 1-butene < 1-hexene ↵
- a) 1-pentene b)3-nonene ↵
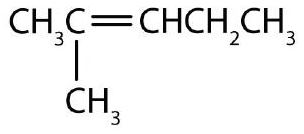 ↵
↵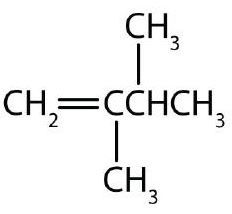 ↵
↵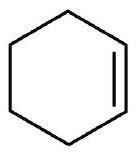 ↵
↵- 1. 2-methyl-1-butene 2. 5-methyl-2-heptene 3. 2,2,4-trimethyl-3-hexene ↵
- Alkenes have double bonds; alkynes have triple bonds. Both undergo addition reactions. ↵
- 4-methyl-1-pentyne ↵
- H–C≡C–H ↵
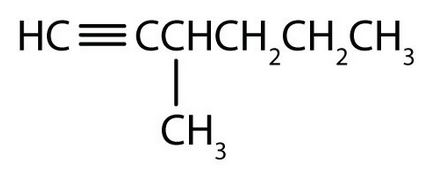 ↵
↵- 1. 1-pentyne 2. 2-hexyne ↵
- Cis-trans isomers are compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule. Alkenes and cyclic compounds can exhibit cis-trans isomerism. ↵
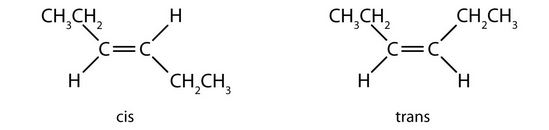
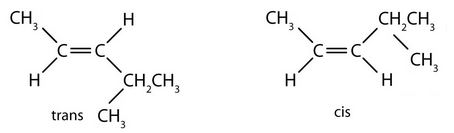
- 1. trans (the two hydrogen atoms are on opposite sides) 2. cis (the two hydrogen atoms are on the same side, as are the two ethyl groups) 3. cis (the two ethyl groups are on the same side) 4. neither (flipping the bond does not change the molecule. There are no isomers for this molecule). ↵
- No; a triply bonded carbon atom can form only one other bond. It would have to have two groups attached to show cis-trans isomerism. ↵
- none. There are two distinct geometric isomers, but since there are there are four different groups off the double bond, these are both cis/trans isomers (they are technically E/Z isomers discussed elsewhere). ↵
 ↵
↵ ↵
↵ ↵
↵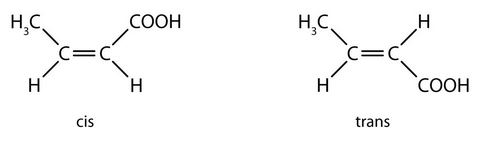 ↵
↵- Alkenes undergo addition reactions; alkanes do not. Both burn. ↵
- C12H24Br2 ↵
- (CH3)2CBrCH2Br ↵
- CH3CH(CH3)CH2CH3 ↵
-
 ↵
↵ - Add bromine solution (reddish-brown) to each. Pentane will not react, and the reddish-brown colour persists; 1-pentene will react, leaving a colourless solution. ↵
- An aromatic compound is any compound that contains a benzene ring or has certain benzene-like properties. ↵
- Valence electrons are shared equally by all six carbon atoms (that is, the electrons are delocalized). ↵
- The six electrons are shared equally by all six carbon atoms. ↵
- meta = 1,3 disubstitution; (answers will vary)
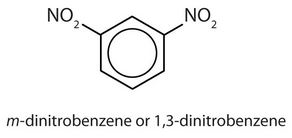 ortho = 1,2 disubstitution
ortho = 1,2 disubstitution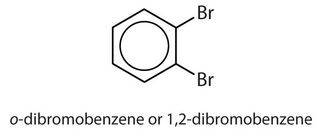
para = 1,4 disubstitution or 1-bromo-4-chlorobenzene
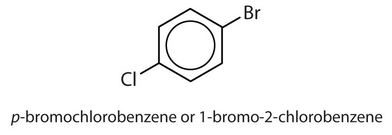 ↵
↵ - phenyl group: C6H5 or
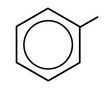
3-phenyloctane:
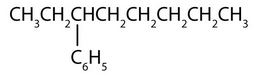 ↵
↵ - a) yes b) no ↵
- a) no b) yes ↵
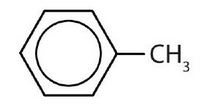 ↵
↵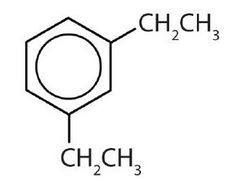 ↵
↵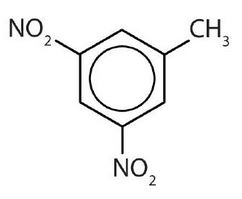 ↵
↵- a) ethylbenzene b) isopropylbenzene c) o-bromotoluene d) 3,5-dichlorotoluene ↵
- a) number not needed b) can’t have two groups on one carbon atom on a benzene ring c) can’t have a substituent on the same carbon atom as the nitro group ↵
- Benzene is rather unreactive toward addition reactions compared to an alkene. ↵

