19.7 Introduction to Green Chemistry
Learning Objectives
By the end of this section, you will be able to:
- Describe the principles of green chemistry
- Outline the general strategy of greening a reaction
Organic chemistry reactions are essential to supporting the world’s economic progress. As a result, the chemical industry is a large user of energy and greenhouse gas emissions. Ethylene, propylene, methanol, and aromatics production account for much of the demands and production. See Compound Interest: The environmental impact of industrial reactions – in C&EN for more details.
What is Green Chemistry?
Green chemistry involves inventing new chemicals, new chemical processes and commercial products that reduce chemical hazards and minimize hazardous effects on human health and the environment. The philosophy is based on 12 principles and are summarized by four key ideas:
- Prevent the formation of waste in the first place.
- Employ safer reagents or solvents.
- Implement selective and efficient transformations (reactions).
- Avoid unnecessary transformations.
For more details on the 12 principles, view Infographic 19.7a.

General Strategy
The process of greening a chemical reaction involves a cyclic process that is outlined in Figure 19.7a. It starts by assessing the existing procedure including the reagents, products, by-products, solvents, reaction conditions, and efficacy. Essentially any components that are used to start, run or are produced in a reaction need to be examined.
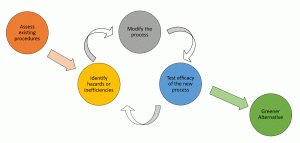
Examples of Green Chemistry
A search of the internet produces many examples of green chemistry. Much of today’s current research in chemistry is focused on green chemistry.
One example is shown in Infographic 19.7b, finding a new source for the production of rubber in tires.
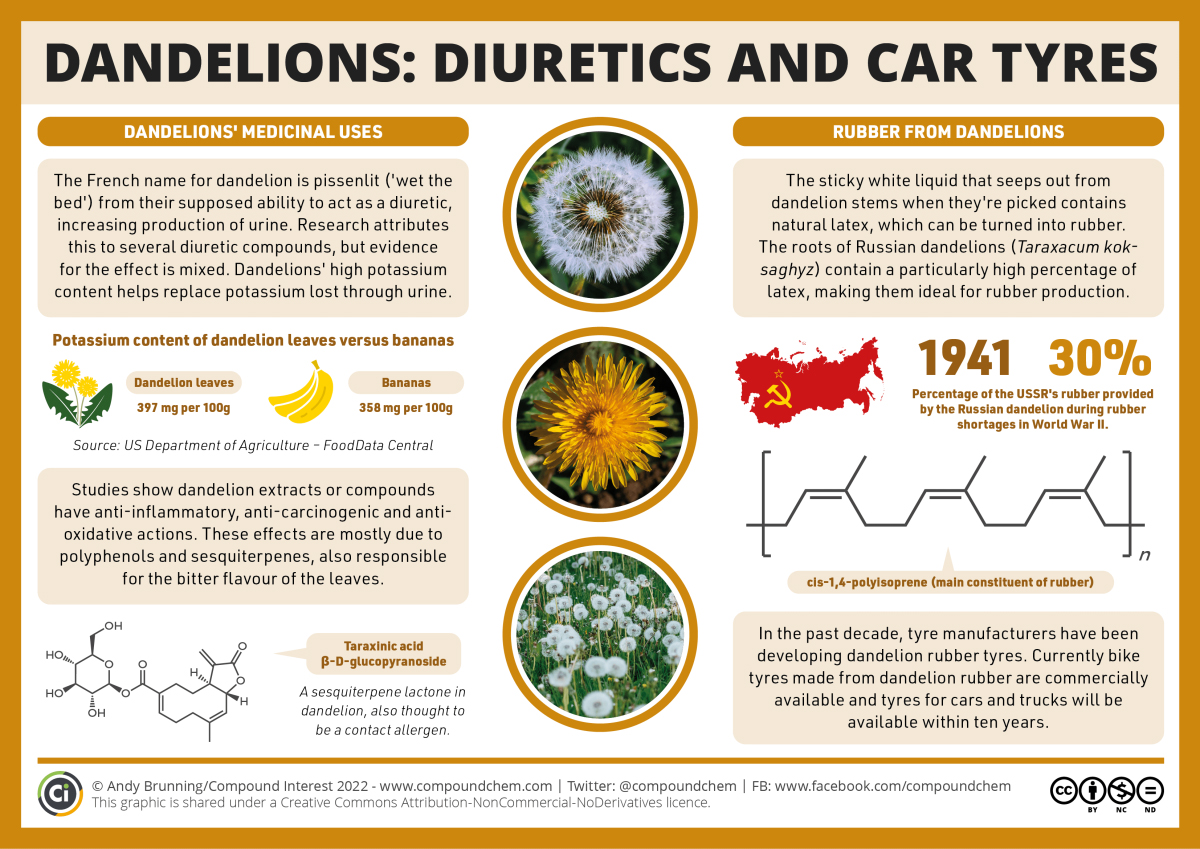
Links to Enhanced Learning
For additional examples, view:
- Examples of Green Chemistry & Sustainable Chemistry – American Chemical Society [New tab]
- Where curiosity and scientific rigor lead to greener chemistry: UNLP-case-study-document-ad-1.pdf [New tab]
- Green Chemistry | US EPA [New tab]
Attribution & References
Except where otherwise noted, this page is written by Samantha Sullivan Sauer and shared under a CC BY-NC 4.0 license.
References
American Chemical Society. (2024). What is green chemistry?
American Chemical Society. (2024). Explore the design principles of green & sustainable chemistry & engineering.
Doxsee, K. M. & Hutchinson, J. E. (2004). Green Organic Chemistry. Thomson: Brooks-Cole.

