Chapter 26 – Review
26.1 Amines – Structure and Naming
- Draw the structure for each compound and classify.
- ethylisopropylamine Check answer[1]
- diethylpropylamine
- Name and classify each compound.
- CH3CH2CH2NH2
- CH3CH2NHCH2CH3
- CH3CH2CH2NHCH3
Check answer[2]
- Draw the structure for each compound and classify.
- Name the following compounds.
-
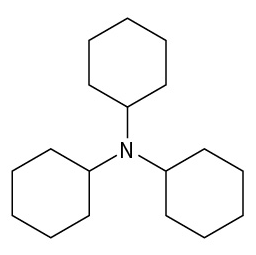
(credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0) Check answer[5]
-
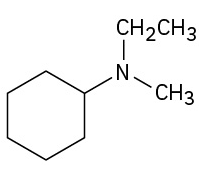
(credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0) Check answer[6]
-
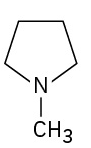
(credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0) Check answer[7]
-
26.2 Amines – Physical Properties
- Which compound of each pair has the higher boiling point? Explain.
- Which compound is more soluble in water—CH3CH2CH3 or CH3CH2NH2? Explain. Check answer[10]
26.3 Heterocyclic Nitrogen Compounds
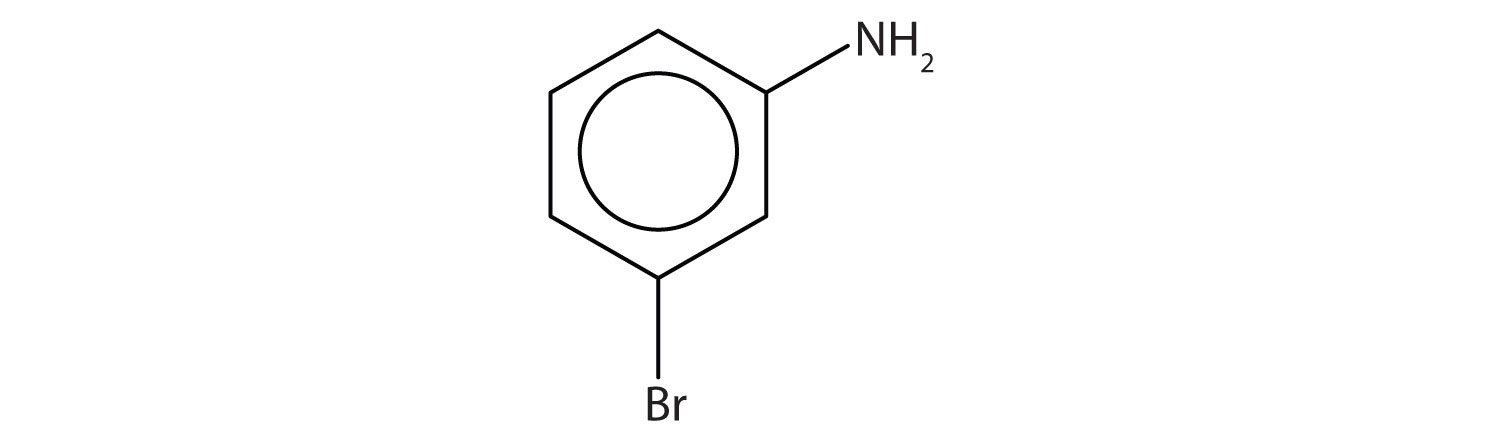
- Name this compound. Check answer[11]
(credit: Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0) - What is a heterocyclic compound? Check answer[12]
26.4 Basicity of Amines
- Explain the basicity of amines. Check answer[13]
- Contrast the physical properties of amines with those of alcohols and alkanes. Check answer[14]
26.5 Amides – Structures, Properties and Naming
-
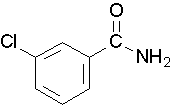
- Draw a structure for the following compound: 3-chlorobenzamide. Check answer[15]
- Try to name the following compound:
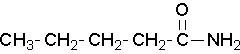
(Image credit: Caryn Fahey/JR van Haarlem) Check answer[16]
- Try to draw a structure for the following compound: N,N-dimethylformamide. Check answer[17]
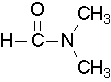
- Try to name the following compound:
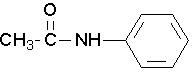
(Image credit: Caryn Fahey/JR van Haarlem) Check answer[18]
- Try to draw a structure for N,N-dimethylformamide.
Check answer[19]
26.6 Chemical Properties of Amines and Amides
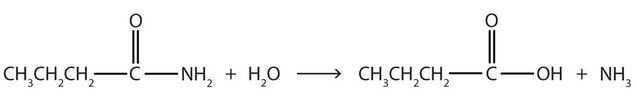
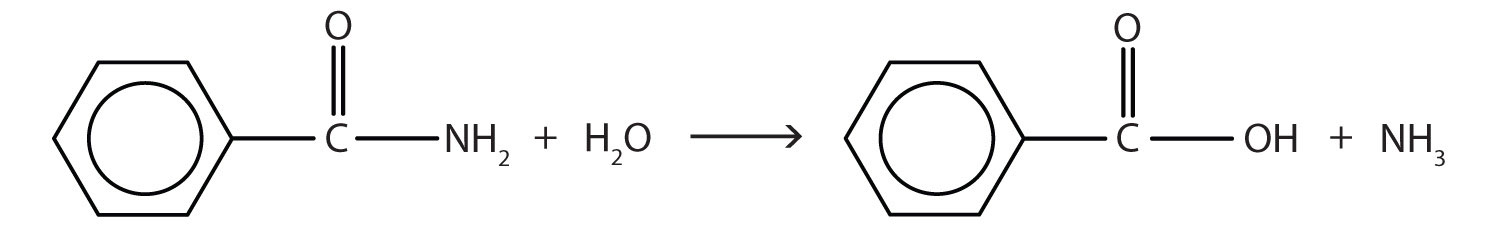
- Write the equation for the hydrolysis of each compound.
- What are the products of the hydrolysis of an amide? Check answer[22]
- When the amide CH3CH2CH2CH2CONH2 is hydrolyzed in an NaOH solution, the products are CH3CH2CH2CH2COO−Na+ and NH3. What products are obtained when CH3CH2CH2CH2CONH2 is hydrolyzed in a hydrochloric acid solution? Check answer[23]
Links to Enhanced Learning
Create your own organic nomenclature quiz to identify, name and draw amines and amides using Organic Nomenclature [New tab]. You can customize the types of questions you receive and get instant feedback.
Find a detailed video here for naming amines Khan Academy – Amines [New tab]
Attribution & References
- a.
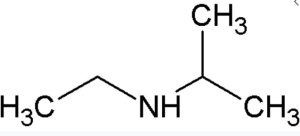 ↵
↵ - a. There is only one alkyl group attached to the nitrogen atom, so the amine is primary. A group of three carbon atoms (a propyl group) is attached to the NH2 group through an end carbon atom, so the name is propylamine, b. There are two ethyl groups attached to the nitrogen atom; the amine is secondary, so the compound is diethylamine, c. The nitrogen atom has a methyl group and a propyl group, so the compound is methylpropylamine, a secondary amine. ↵
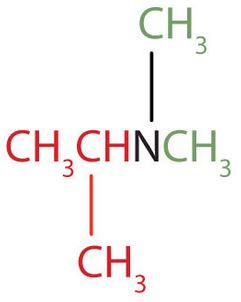 The name indicates that there are an isopropyl group (in red) and two methyl groups (in green) attached to the nitrogen atom; the amine is tertiary. ↵
The name indicates that there are an isopropyl group (in red) and two methyl groups (in green) attached to the nitrogen atom; the amine is tertiary. ↵- b. The name indicates that there are two propyl groups attached to the nitrogen atom; the amine is secondary. (The third bond on the nitrogen atom goes to a hydrogen atom.) CH3CH2CH2NHCH2CH2CH3 ↵
- tricyclohexylamine ↵
- N-ethyl-N-methylcyclohexylamine ↵
- N-Methylpyrrolidine ↵
- butylamine because the N–H bonds can engage in hydrogen bonding; pentane cannot engage in hydrogen bonding ↵
- CH3CH2CH2CH2CH2NH2 because it has a greater molar mass than CH3NH2 ↵
- CH3CH2NH2 because amines can engage in hydrogen bonding with water; alkanes cannot engage in hydrogen bonding ↵
- The benzene ring with an amino (NH2) group is aniline. The compound is named as a derivative of aniline: 3-bromoaniline or m-bromoaniline. ↵
- Heterocyclic compounds are ring compounds with atoms other than carbon atoms in the ring. ↵
- Amines have a lone pair of electrons on the nitrogen atom and can thus act as proton acceptors (bases). ↵
- The solubilities of amines are similar to those of alcohols; the boiling points of primary and secondary amines are similar to those of alcohols; the boiling points of tertiary amines, which cannot engage in hydrogen bonding because they do not have a hydrogen atom on the nitrogen atom, are comparable to those of alkanes. ↵
 ↵
↵- pentamide ↵
-
 ↵
↵ - N-phenylethanamide or N-phenylacetamide ↵
 ↵
↵- The hydrolysis of a simple amide produces an organic acid and ammonia. Butyramide thus yields butyric acid and ammonia.
 ↵
↵ - The hydrolysis of an amide produces an organic acid and ammonia. Benzamide thus yields benzoic acid and ammonia.
 ↵
↵ - a carboxylic acid and ammonia or an amine ↵
- CH3CH2CH2CH2COOH and NH4Cl ↵

