Chapter 24: Aldehydes and Ketones
Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry
by Gregory Anderson; Jen Booth; Caryn Fahey; Adrienne Richards; Samantha Sullivan Sauer; and David Wegman
Chapter 24 Contents
In this chapter, you will learn about:
- The carbonyl functional group, and the structures of aldehydes and ketones
- Naming aldehydes and ketones
- Physical and chemical properties of aldehydes and ketones
- Common aldehydes and ketones
Except where otherwise noted, this OER is licensed under CC BY-NC-SA 4.0
Please visit the web version of Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry to access the complete book, interactive activities and ancillary resources.
To better support your learning, you should be familiar with the following concepts before starting this chapter:
- sp2–sp2 hybridization, sigma and pi bonding to form double bonds in organic molecules (Chapter 21.1 Valence Bond Theory)
- General nomenclature rules for naming alkanes (Chapter 20.3: Isomers of Alkanes and IUPAC Nomenclature)
- The difference between a physical and a chemical property of matter

Aside from carbon, which is found in all organic compounds, one of the most common atoms found in organic molecules is oxygen. When carbon double bonds with an oxygen atom, this grouping of atoms (termed a “carbonyl group”) is very reactive and is the functional group that is always found in both aldehydes and ketones. The Formica tabletops that are found at most diners, the adhesives that are used to manufacture the plywood used to make your shed, and the formaldehyde used to preserve animal specimens in a science lab are all examples of common aldehydes. Similarly, the main component of most nail polish removers, acetone, is one of the simplest and most common ketones that we encounter regularly. But aldehydes and ketones are not all harsh chemicals – many spices and flavouring agents that we eat daily belong to these two groups of organic molecules. Cinnamon, vanilla, and the characteristic flavour of almonds are all due to the presence of aldehyde molecules. And that butter flavour in your microwave popcorn, or the distinctive flavour of blue cheese? These are due to the presence of ketones.
From a biochemistry standpoint, aldehydes and ketones are essential to life on Earth. Carbohydrates exist as aldehydes and ketones: the special reactivity of aldehydes and ketones allow starches and cellulose to form long polymers, which allow both plants and animals to store energy long-term. DNA, which is the genetic code for all living things on this planet, is bonded in place by carbonyl group chemical reactions: the genetic code of adenine, guanine, cytosine, and thymine is connected to a “backbone” which is provided by the carbonyl group.
Spotlight on Everyday Chemistry: The smell of wet dog
Those who have a dog know that when wet, many dogs have a unique smell. Many of the compounds that result in the odour are based on the carbonyl functional group in the form of aldehydes and ketones. Infographic 24.0a. highlights some of the key compounds that make the smell of wet dog.
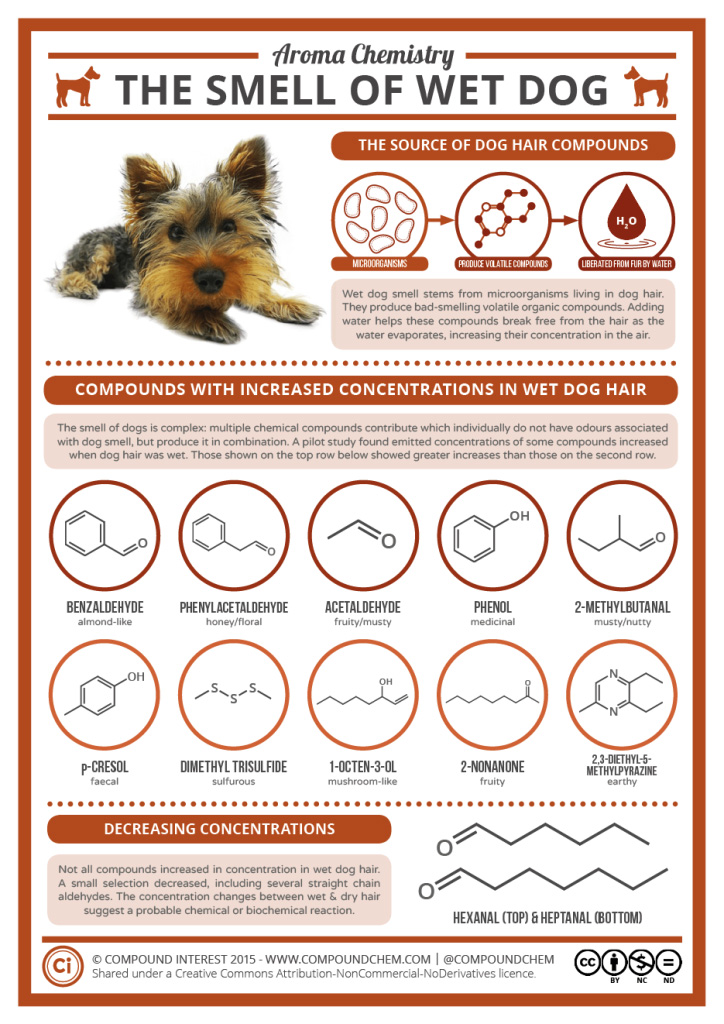
Watch the first portion of An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27 (youtube.com) [11 min] to learn more about aldehydes and ketones. (Later portions of the video are not relevant to this text.)
Video Source: Crash Course. (2021, April 28). An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27 (youtube.com) [Video]. YouTube.
Attribution & References
Except where otherwise noted, this page is adapted by Gregory A. Anderson and Samantha Sullivan Sauer from “15: Aldehydes and Ketones” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), a derivative of Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0 by LibreTexts, licensed under CC BY-NC-SA 3.0. Attribution from original source: “16.5: Other Oxygen-Containing Functional Groups” In Beginning Chemistry (v. 1.0) by Anonymous is licensed CC BY-NC-SA 3.0.

