Chapter 24 – Review
24.1 The Carbonyl Group
- What are some similarities and differences between aldehydes and ketones?
- What is the smallest aldehyde? What is the smallest ketone? Check answer[1]
- Draw an aldehyde and a ketone each having five carbons. There are multiple acceptable answers.
24.2 Naming Aldehydes and Ketones
- Draw the structure of a) 5-bromo-3-iodoheptanal, and b) 5-bromo-4-ethyl-2-heptanone.
- Give the structure and IUPAC name for the compound that has the common name m-bromobenzaldehyde. Check answer[2]
- Give the IUPAC name for glyceraldehyde. (Hint: There are two functional groups on the same molecule. Aldehydes take priority over -OH groups. Keep the suffix -al, but use hydroxy to name the alcohol as the lower priority substituent. As a substituent, the OH group is named hydroxy.)
(credit: Intro Biochem, CC BY-NC-SA 4.0). Check answer[3]
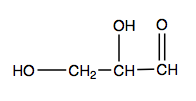
- Name each compound by IUPAC nomenclature.
(credit: Intro Biochem, CC BY-NC-SA 4.0). Check answer[4]
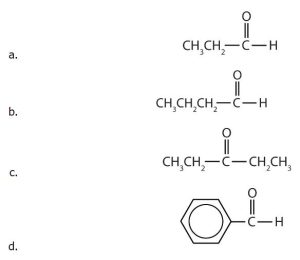
- Name each compound by IUPAC nomenclature.
(credit: Intro Biochem, CC BY-NC-SA 4.0). - Draw the structure for each compound. a) butanal, b) 2-hexanone, c) 5-ethyloctanal, d) 2-chloropropanal, e) 2-hydroxy-3-pentanone.
24.3 Physical Properties of Aldehydes and Ketones
- Which compound in each pair has the higher boiling point? a) propanone or 2-propanol b) methoxymethane or ethanal Check answer[5]
- Which compound in each pair has the higher boiling point? a) butanal or 1-butanol b) acetone (propanone) or 2-methylpropane
- Describe the solubility of aldehydes and ketones compared to alkanes.
- There are many examples of aldehydes and ketones used in nature and in everyday life. Do some internet research to find one or two more examples.
24.4 Chemical Properties of Aldehydes and Ketones
- What feature of their structure makes aldehydes easier to oxidize than ketones? Check answer[6]
- How does the carbon-to-oxygen bond of aldehydes and ketones differ from the carbon-to-carbon bond of alkenes? Check answer[7]
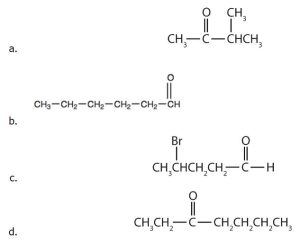
- Order the following molecules from least to most oxidized, based on the marked carbon atom:
(credit: Chemistry (OpenStax), CC BY 4.0) - Explain why it is not possible to prepare a ketone that contains only two carbon atoms. Check answer[8]
- How does hybridization of the substituted carbon atom change when an alcohol is converted into an aldehyde? An aldehyde to a carboxylic acid?
- Predict the products of oxidizing the molecules shown in this problem. In each case, identify the product that will result from the minimal increase in oxidation state for the highlighted carbon atom:(a)
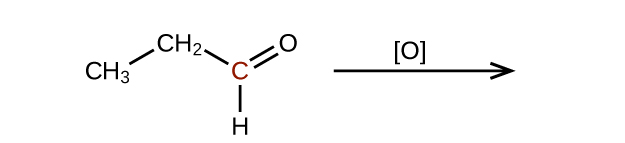 Check answer[9]
Check answer[9]
(b)
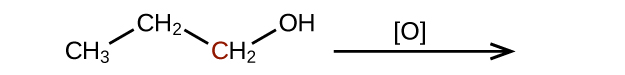
(credit: Chemistry (OpenStax), CC BY 4.0) Check answer[10]
(c)
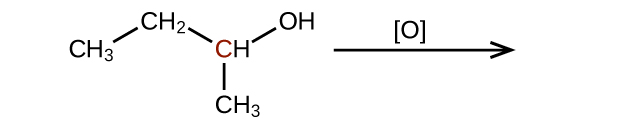
(credit: Chemistry (OpenStax), CC BY 4.0) Check answer [11]
- Predict the products of reducing the following molecules. In each case, identify the product that will result from the minimal decrease in oxidation state for the highlighted carbon atom:
(a)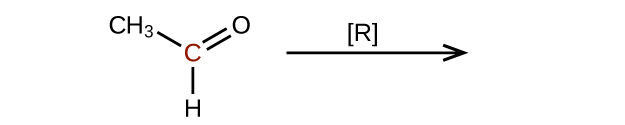
(credit: Chemistry (OpenStax), CC BY 4.0) (b)
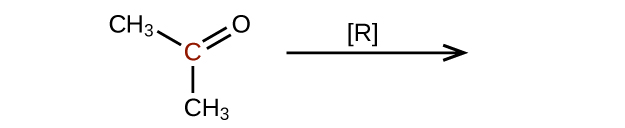
(credit: Chemistry (OpenStax), CC BY 4.0) 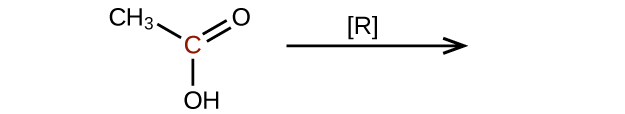
(credit: Chemistry (OpenStax), CC BY 4.0) - Ethanal is treated with each substance. a) Ag+(aq) — What change can be seen visually? b) [O] — What organic product, if any, is formed? Check answer[12]
- Acetone (propanone) is treated with each substance. a) Ag+(aq) — What change can be seen visually? b) [O] in an acid solution — What organic product, if any, is formed?
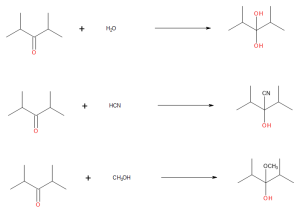
- Draw the products from these addition reactions.
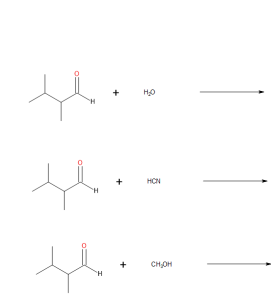
(credit: Samantha Sullivan Sauer / Biovia Draw) - Draw the products from these addition reactions.
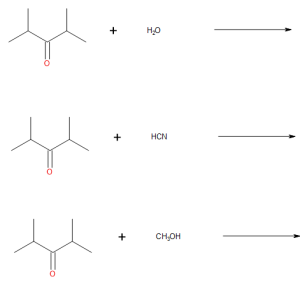
(credit: Samantha Sullivan Sauer / Biovia Draw) Check answer[13]
Links to Enhanced Learning
Create your own organic nomenclature quiz to identify, name and draw aldehydes and ketones using Organic Nomenclature (orgchem101.com). You can customize the types of questions you receive and get instant feedback.
Attribution & References
Except where otherwise noted, this page (including images in solutions) is written and adapted by Gregory A. Anderson and Samantha Sullivan Sauer from
- “18.3 Aldehydes, Ketones, Carboxylic Acids, and Esters” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “14.9: Aldehydes and Ketones- Structure and Names” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- “4.1: Aldehydes and Ketones- Structure and Names” and “4.2: Properties of Aldehydes and Ketones” In Introductory Biochemistry by LibreTexts, CC BY-NC-SA 4.0
- aldehyde - methanal (or formaldehyde); ketone - propanone (or acetone) ↵
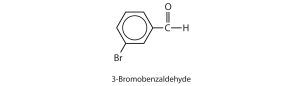 3-bromobenzaldehyde ↵
3-bromobenzaldehyde ↵- 2,3-dihydroxypropanal ↵
- a) propanal, b) butanal, c) 3-pentanone, d) benzaldehyde ↵
- a) 2-propanol, b) ethanal ↵
- the H on the carbonyl carbon atom ↵
- The carbon-to-oxygen double bond is polar; the carbon-to-carbon double bond is nonpolar. ↵
- A ketone contains a group bonded to two additional carbon atoms; thus, a minimum of three carbon atoms are needed. ↵
-
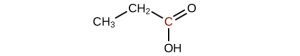 ↵
↵ -
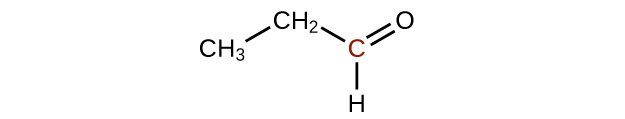 ↵
↵ -
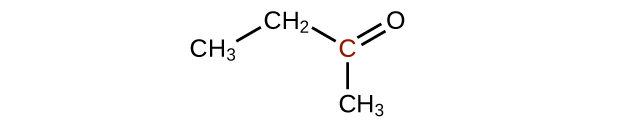 ↵
↵ - a) silver metal (Ag) on reaction vessel, b) ethanoic acid ↵
-
 ↵
↵

