25.5 Esters – Structure, Properties and Naming
Learning Objectives
By the end of this section, you will be able to:
- Identify the general structure for an ester.
- Use common names to name esters.
- Name esters according to the IUPAC system.
- Compare the boiling points of esters with alcohols of similar molar mass.
- Compare the solubilities of esters in water with the solubilities of comparable alkanes and alcohols in water.
Esters contain a carbonyl group with a second oxygen atom bonded to the carbon atom in the carbonyl group by a single bond. In an ester, the second oxygen atom bonds to another carbon atom (Figure 25.5a.). The names for esters include prefixes that denote the lengths of the carbon chains in the molecules and are derived following nomenclature rules similar to those for inorganic acids and salts. The functional groups for an ester are shown in red below.
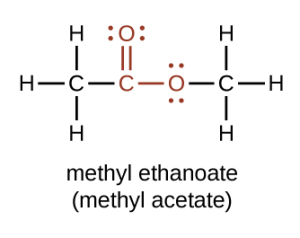
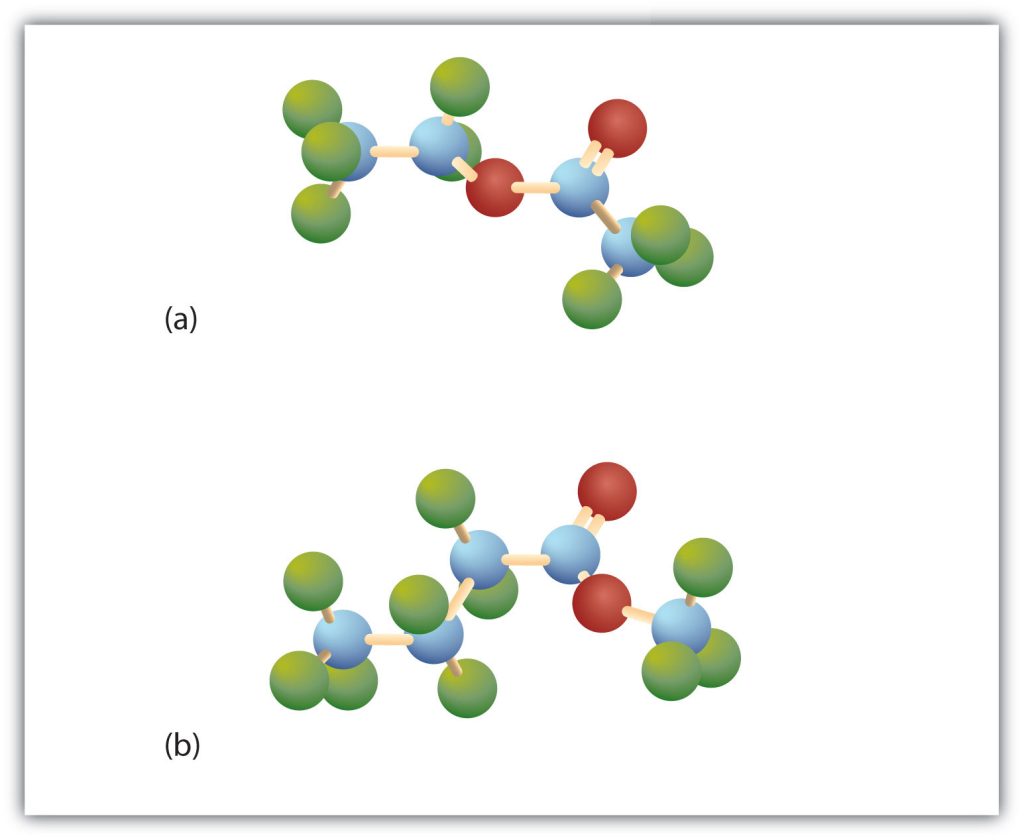
Esters have the general formula RCOOR′, where R may be a hydrogen atom, an alkyl group, or an aryl group, and R′ may be an alkyl group or an aryl group but not a hydrogen atom. (If it were hydrogen atom, the compound would be a carboxylic acid.) Figure 25.5b. shows models for two common esters.
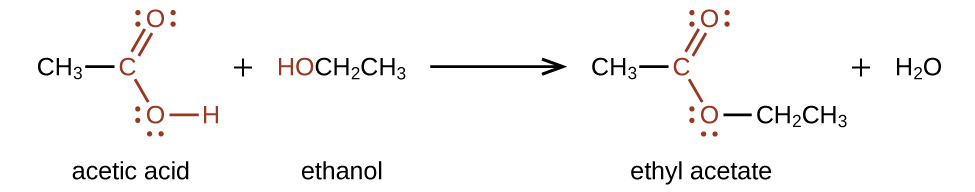
Esters are produced by the reaction of acids with alcohols. For example, the ester ethyl acetate, CH3CO2CH2CH3, is formed when acetic acid reacts with ethanol (Figure 25.5c.).
Properties of Esters
Esters occur widely in nature. Unlike carboxylic acids, esters generally have pleasant odours and are often responsible for the characteristic fragrances of fruits and flowers. Once a flower or fruit has been chemically analyzed, flavour chemists can attempt to duplicate the natural odour or taste. Both natural and synthetic esters are used in perfumes and as flavouring agents. Fats and vegetable oils are esters of long-chain fatty acids and glycerol. Esters of phosphoric acid are of the utmost importance to life.
Ester molecules are polar but have no hydrogen atom attached directly to an oxygen atom. They are therefore incapable of engaging in intermolecular hydrogen bonding with one another and thus have considerably lower boiling points than their isomeric carboxylic acids counterparts. Because ester molecules can engage in hydrogen bonding with water molecules, however, esters of low molar mass are somewhat soluble in water. Borderline solubility occurs in those molecules that have three to five carbon atoms. Table 25.5a. lists the physical properties of some common esters.
Esters are common solvents. Ethyl acetate is used to extract organic solutes from aqueous solutions—for example, to remove caffeine from coffee. It also is used to remove nail polish and paint. Cellulose nitrate is dissolved in ethyl acetate and butyl acetate to form lacquers. The solvent evaporates as the lacquer “dries,” leaving a thin film on the surface. High boiling esters are used as softeners (plasticizers) for brittle plastics.
| Condensed Structural Formula | Name | Molar Mass | Melting Point (°C) | Boiling Point (°C) | Aroma |
|---|---|---|---|---|---|
| HCOOCH3 | methyl formate | 60 | −99 | 32 | |
| HCOOCH2CH3 | ethyl formate | 74 | −80 | 54 | rum |
| CH3COOCH3 | methyl acetate | 74 | −98 | 57 | |
| CH3COOCH2CH3 | ethyl acetate | 88 | −84 | 77 | |
| CH3CH2CH2COOCH3 | methyl butyrate | 102 | −85 | 102 | apple |
| CH3CH2CH2COOCH2CH3 | ethyl butyrate | 116 | −101 | 121 | pineapple |
| CH3COO(CH2)4CH3 | pentyl acetate | 130 | −71 | 148 | pear |
| CH3COOCH2CH2CH(CH3)2 | isopentyl acetate | 130 | −79 | 142 | banana |
| CH3COOCH2C6H5 | benzyl acetate | 150 | −51 | 215 | jasmine |
| CH3CH2CH2COO(CH2)4CH3 | pentyl butyrate | 158 | −73 | 185 | apricot |
| CH3COO(CH2)7CH3 | octyl acetate | 172 | −39 | 210 | orange |
Source: “15.6: Physical Properties of Esters” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
Spotlight of Everyday Chemistry: Esters and Their Smells
The infographic below represents some of the common smells produced by esters. We find that several fruity smells we are familiar with in the foods we eat and cook with are produced by esters.

The distinct smell we can imagine when we walk through a Christmas tree farm is due to an ester found in the oils of conifer trees. Similar to Christmas trees, mangoes have a distinct aroma. This smell is due to an ester which provides the fruity notes found in mangoes.
Visit the Compound Interest website by Andy Brunning to read more about the “Aroma Chemistry: The Aroma of Christmas Trees [New tab]” or the “The Chemistry of Mangos: What Do They Have in Common with Poison Ivy? [New tab]”
Names of Esters
Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming salts.
Here are some basic rules for naming esters from the International Union of Pure and Applied Chemistry (IUPAC):
- Write the name for the carbon chain from the alcohol as an alkyl group. For example if the alcohol is ethanol, the first word in ester naming will be ethyl.
- The second word for naming an ester involves naming the acid but substituting the -oic ending for -oate. For example if the acid is ethanoic acid, the second word in the name becomes ethanoate.
The group name of the alkyl or aryl portion is given first and is followed by the name of the acid portion. In both common and International Union of Pure and Applied Chemistry (IUPAC) nomenclature, the –ic ending of the parent acid is replaced by the suffix –ate (Table 25.5b.).
| Condensed Structural Formula | Common Name | IUPAC Name |
|---|---|---|
| HCOOCH3 | methyl formate | methyl methanoate |
| CH3COOCH3 | methyl acetate | methyl ethanoate |
| CH3COOCH2CH3 | ethyl acetate | ethyl ethanoate |
| CH3CH2COOCH2CH3 | ethyl propionate | ethyl propanoate |
| CH3CH2CH2COOCH(CH3)2 | isopropyl butyrate | isopropyl butanoate |
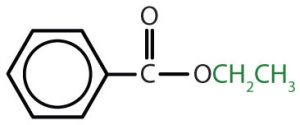 |
ethyl benzoate | ethyl benzoate |
Table and image credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0.
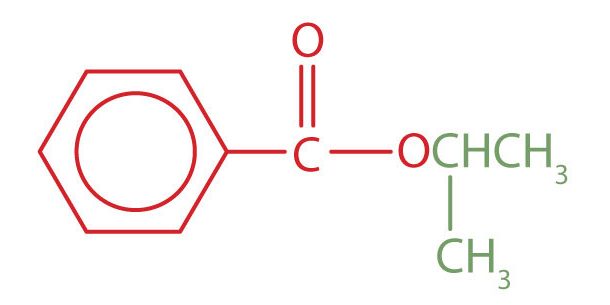
Example 25.5a
Give the common and IUPAC names for each compound.

Solution:
a. The alkyl group attached directly to the oxygen atom is a butyl group (in green).

The part of the molecule derived from the carboxylic acid (in red) has three carbon atoms. It is called propionate (common) or propanoate (IUPAC). The ester is therefore butyl propionate or butyl propanoate.
b. An alkyl group (in green) is attached directly to the oxygen atom by its middle carbon atom; it is an isopropyl group. The part derived from the acid (that is, the benzene ring and the carbonyl group, in red) is benzoate. The ester is therefore isopropyl benzoate (both the common name and the IUPAC name).
Exercise & Image credits: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0
Exercise 25.5a
Give the common and IUPAC names for each compound.

Check Your Answers:[1]
Exercise & Image credits: Introduction to Chemistry: GOB., CC BY-NC-SA 3.0.
Exercise 25.5b
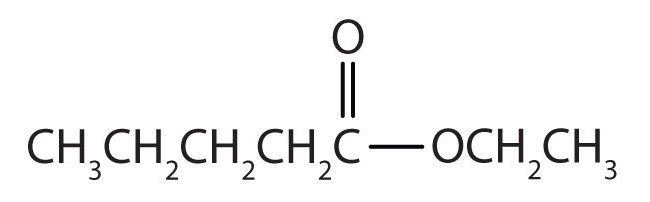
Draw the structure for ethyl pentanoate.
Check Your Answer[2]
Exercise & solution image credits: Introduction to Chemistry: GOB., CC BY-NC-SA 3.0.
Indigenous Perspectives: The Strawberry
The strawberry or ken’niiohontésha in Mohawk language, is a symbol of importance in woman’s medicine and for naming babies in the longhouse. The strawberry is one of several festivals in Haudenosaunee’s cycle of ceremonies to give thanks to the natural world.

For more details on the importance of the strawberry read: CBC News – Strawberry harvest has cultural and ceremonial significance for Kahnawake community [New tab]. The interview below with Elder Duke Redbird also looks at the importance of the strawberry “heart berry”: City News – The Indigenous story of the Strawberry Moon [New tab].
Spotlight on Everyday Chemistry: Julian Silverman’s Research on Oil Spill Cleanup


Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey from:
- “18.3 Aldehydes, Ketones, Carboxylic Acids, and Esters” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “15.5: Esters – Structures and Names” & “15.6: Physical Properties of Esters” In Basics of General, Organic, and Biological Chemistry (Ball et al.)by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
-
a. ethyl pentanoate, b. propyl propanoate.
↵
-
Start with the portion from the acid. Draw the pentanoate (five carbon atoms) group first; keeping in mind that the last carbon atom is a part of the carboxyl group.
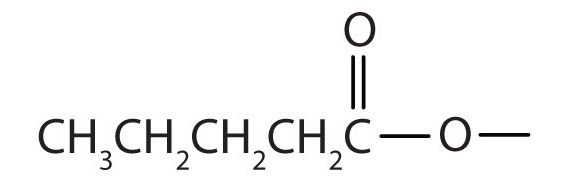 Then attach the ethyl group to the bond that ordinarily holds the hydrogen atom in the carboxyl group.
Then attach the ethyl group to the bond that ordinarily holds the hydrogen atom in the carboxyl group.
↵
organic compound containing a carbonyl group with an attached oxygen atom that is bonded to a carbon substituent

