23.7 Thiols
Learning Objectives
By the end of this section, you will be able to:
- Identify and name thiols (mercaptans) by the presence of an SH group.
- Identify sulfides and disulfides.
- Understand that the mild oxidation of thiols gives disulfides.
- Recognize importance of sulfur in organic compounds.
Thiol Structure
Because sulfur is in the same group (6A) of the periodic table as oxygen, the two elements have some similar properties. We might expect sulfur to form organic compounds related to those of oxygen, and indeed it does.
Thiols (also called mercaptans), which are sulfur analogs of alcohols, have the general formula RSH. Methanethiol (also called methyl mercaptan), has the formula CH3SH. Ethanethiol (ethyl mercaptan) is the most common odourant for liquid propane (LP) gas. Some other thiols are shown in Figure 23.7a. Because of the lack of the ability to hydrogen bond, thiols have lower boiling points than the corresponding alcohols. For example, ethanethiol is a gas at room temperature.
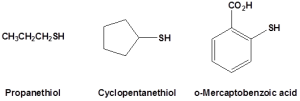
The most striking characteristic of thiols is their appalling odour. Skunk scent, for instance, is caused primarily by the simple thiols 3-methyl-1-butanethiol and 2-butene-1-thiol. Volatile thiols such as ethanethiol are also added to natural gas and liquefied propane to serve as an easily detectable warning in case of leaks.
Naming Thiols
Rules for naming thiols are similar to rules for naming alcohols.
- Thiols are named by adding the word –thiol as the suffix of the parent name.
- The longest carbon chain is then numbered to give the sulfhydryl group the lowest possible number.
- Since the functional group has priority in numbering, if the sulhydryl (-SH) group is bonded to a ring, the carbon that it is bonded to is assigned to C1 and the number is omitted from the name.
Example 23.7a
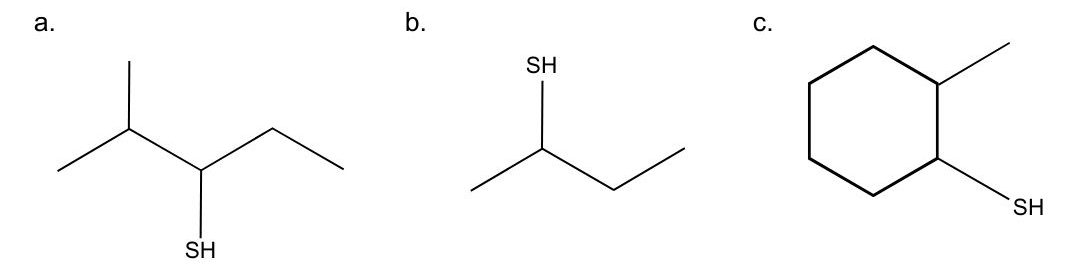
What is the name of each thiol?
Solution:
- This molecule is named 2-methyl-3-pentanethiol (or 2-methylpentane-3-thiol).
- The longest continuous chain of carbon has four carbon atoms, so the stem name is butane. The parent name is obtained by adding the word thiol, to give butanethiol. We number from the left to give the sulfhydryl group the lowest number. Since there are no substituents present, the name of the molecule is 2-butanethiol (or butane-2-thiol).
- The longest continuous chain of carbon has six carbon atoms in a ring, so the stem name is cyclohexane. The parent name is obtained by adding the word thiol, to give cyclohexanethiol. The carbon that has the sulfhydryl group is assigned as C1, since any carbon in the ring can be C1. The ring is then number counterclockwise to give the methyl substituent the lowest possible number. Therefore, the name of the molecule is 2-methylcyclohexanethiol.
Exercise 23.7a
Name the following molecules.
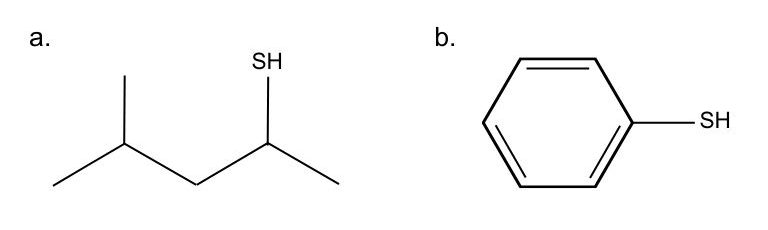
Check Your Answers:[1]
Sulfides and Disulfides
Sulfides, also called thioethers, have the structure R-S-R’ and are sulfur analogs of ethers. Disulfides have the structure R-S-S-R’. These are both commonly found in biomolecules. Dimethylsulfide (CH3SCH3) which is responsible for the sometimes unpleasant odour of cooking cabbage and related vegetables.
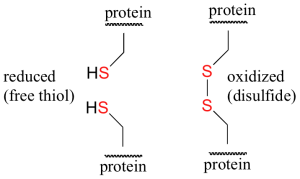
Oxidation of thiols and other sulfur compounds changes the oxidation state of sulfur rather than carbon. The mild oxidation of thiols gives disulfides (Figure 23.7b.). An equivalent oxidation of alcohols to peroxides is not normally observed. The reasons for this different behaviour are not hard to identify. The S–S single bond is nearly twice as strong as the O–O bond in peroxides, and the O–H bond is more than 25 kcal/mole stronger than an S–H bond. Thus, thermodynamics favours disulfide formation over peroxide.
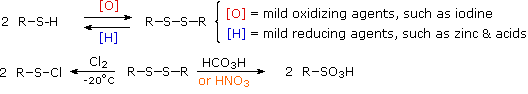
The amino acids cysteine [HSCH2CH(NH2)COOH] and methionine [CH3SCH2CH2CH(NH2)COOH] contain sulfur atoms, as do all proteins that contain these amino acids. Disulfide linkages (–S–S–) between protein chains are extremely important in protein structure.
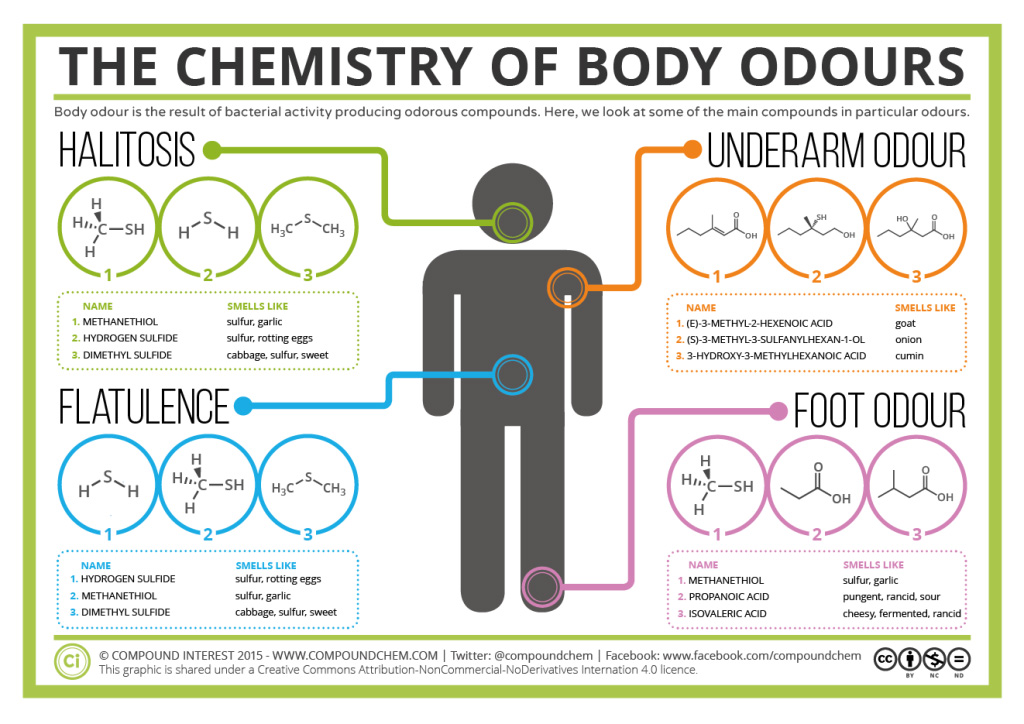
Spotlight on Everyday Chemistry: Body Odours
Many body odours are the result of organic based sulfur compounds. Halitosis (bad breath), underarm odour, flatulence and foot odour are caused by bacterial activity generating mostly organosulfur compounds. Read more in Infographic 23.7a.
Links to Enhanced Learning
Read more about the use of thiols, sulfides and disulfides in everyday compounds.
Food:
- Compound Interest: The Chemistry of Eggs & Egg Shells [New tab]
- Compound Interest: The Chemistry of Camembert [New tab]
- Compound Interest: Aroma Chemistry – The Smell of Freshly-Baked Bread [New tab]
- Compound Interest: Blackcurrants & Cat Urine – The Chemistry of Blackcurrants [New tab]
- Compound Interest: The Chemistry of Brussels Sprouts: Bitterness & Genetics [New tab]
- Compound Interest: What Compounds Cause Garlic Breath? – The Chemistry of Garlic [New tab]
- Compound Interest: The Chemistry of an Onion [New tab]
- Compound Interest: Why Does Asparagus Make Urine Smell? – The Chemistry of Asparagus [New tab]
Waste:
Attribution & References
- a) 4-methylpentane-2-thiol, b) benzenethiol or thiophenol ↵
sulfur analogs of alcohols, have the general formula RSH. also called mercaptans

