23.2 Physical Properties of Alcohols
Learning Objectives
By the end of this section, you will be able to:
- Explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses.
- Explain why alcohols and ethers of four or fewer carbon atoms are soluble in water while comparable alkanes are not soluble.

Like the H–O–H bond in water, the R–O–H bond is bent, and alcohol molecules are polar (Figure 23.2b.).
Boiling Point
The relationship between the structure of water and the structure of alcohols is particularly apparent in small molecules and reflected in the physical and chemical properties of alcohols with low molar mass. Replacing a hydrogen atom from an alkane with an OH group allows the molecules to associate through hydrogen bonding (Figure 23.2c.).
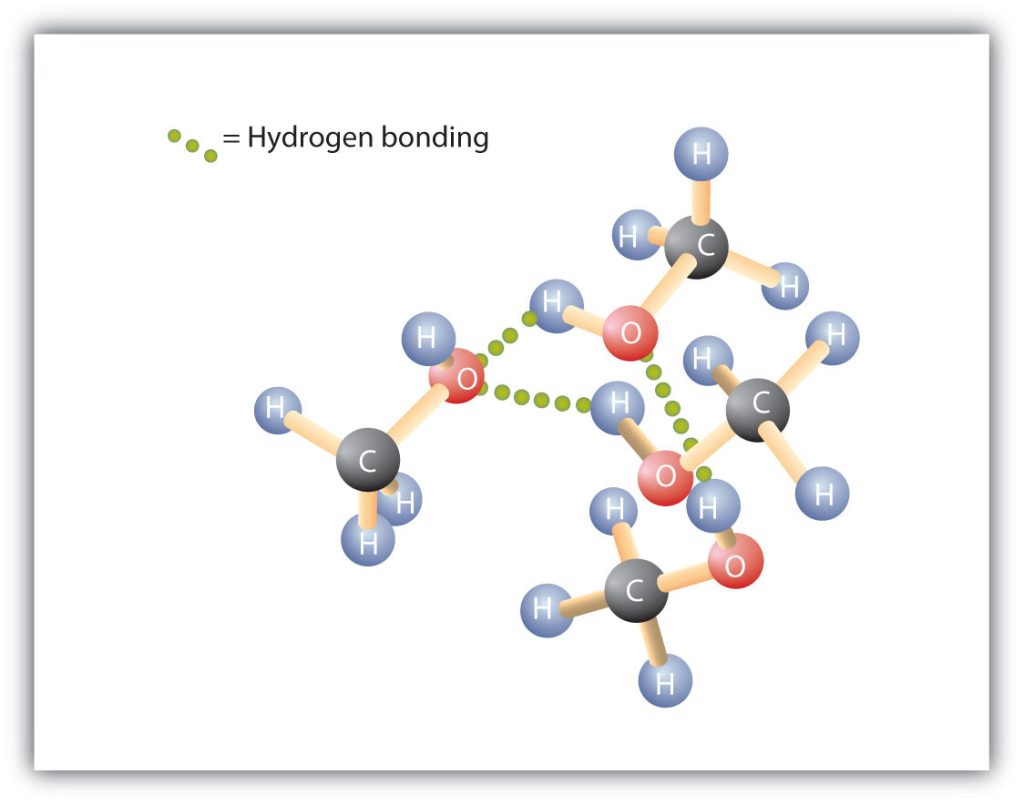
Recall that physical properties are determined to a large extent by the type of intermolecular forces. Table 23.2a. lists the molar masses and the boiling points of some common compounds. The table shows that substances with similar molar masses can have quite different boiling points.
| Formula | Name | Molar Mass | Boiling Point (°C) |
|---|---|---|---|
| CH4 | methane | 16 | –164 |
| HOH | water | 18 | 100 |
| C2H6 | ethane | 30 | –89 |
| CH3OH | methanol | 32 | 65 |
| C3H8 | propane | 44 | –42 |
| CH3CH2OH | ethanol | 46 | 78 |
| C4H10 | butane | 58 | –1 |
| CH3CH2CH2OH | 1-propanol | 60 | 97 |
Alkanes are nonpolar and are thus associated only through relatively weak dispersion forces. Alkanes with one to four carbon atoms are gases at room temperature. In contrast, even methanol (with one carbon atom) is a liquid at room temperature. Hydrogen bonding greatly increases the boiling points of alcohols compared to hydrocarbons of comparable molar mass. The boiling point is a rough measure of the amount of energy necessary to separate a liquid molecule from its nearest neighbours. If the molecules interact through hydrogen bonding, a relatively large quantity of energy must be supplied to break those intermolecular attractions. Only then can the molecule escape from the liquid into the gaseous state.
Adding additional -OH groups to an alcohol molecule will increase the boiling point because there are more opportunities for hydrogen bonding. Consider propan-1-ol (CH3CH2CHOH) which has a molar mass of 60 g/mol and a boiling point of 97oC. Now consider 1,2-ethanediol, also known as ethylene glycol, which has a molar mass of 62 g/mol and a boiling point of 197oC. The presence of one additional -OH group significantly increase the hydrogen bonding ability and as such the boiling point (National Center for Biotechnology Information, 2024a,b).
Ethylene glycol is the main ingredient in many antifreeze mixtures for automobile radiators. Because of its high boiling point, ethylene glycol does not boil away when it is used as an antifreeze. It is also completely miscible with water. A solution of 60% ethylene glycol in water freezes at −49°C (−56°F) and thus protects an automobile radiator down to that temperature.
Solubility
Alcohols can also engage in hydrogen bonding with water molecules (Figure 23.2d.). Thus, whereas the hydrocarbons are insoluble in water, alcohols with one to three carbon atoms are completely soluble.
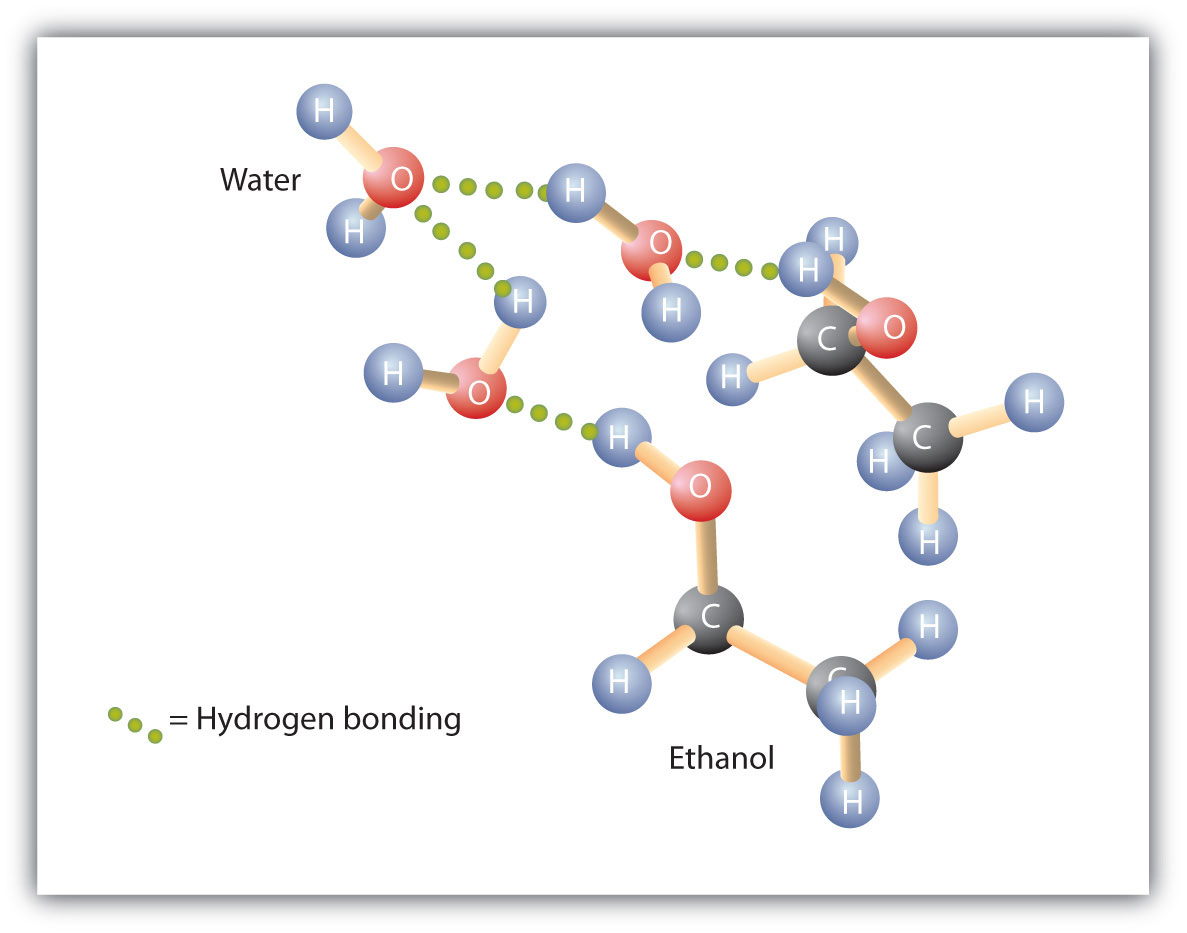
As the length of the chain increases, however, the solubility of alcohols in water decreases; the molecules become more like hydrocarbons and less like water. The alcohol 1-decanol (CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2OH) is essentially insoluble in water. We frequently find that the borderline of solubility in a family of organic compounds occurs at four or five carbon atoms.
Adding additional -OH groups to an alcohol molecule will increase the solubility because there are more opportunities for hydrogen bonding with water. Consider hexan-1-ol (CH3CH2CH2CH2CH2CH2OH) which has a molar mass of 102 g/mol and a solubility of 5.9 g/L at 25oC. Now consider D-glucose, also known as 2,3,4,5,6-pentahydroxyhexanal (see Figure 23.1b), which has a molar mass of 180 g/mol and a solubility of 909 g/L at 25oC. The presence of additional -OH groups significantly increase the hydrogen bonding ability with water and as such the solubility (National Center for Biotechnology Information, 2024c; “Glucose”, 2023).
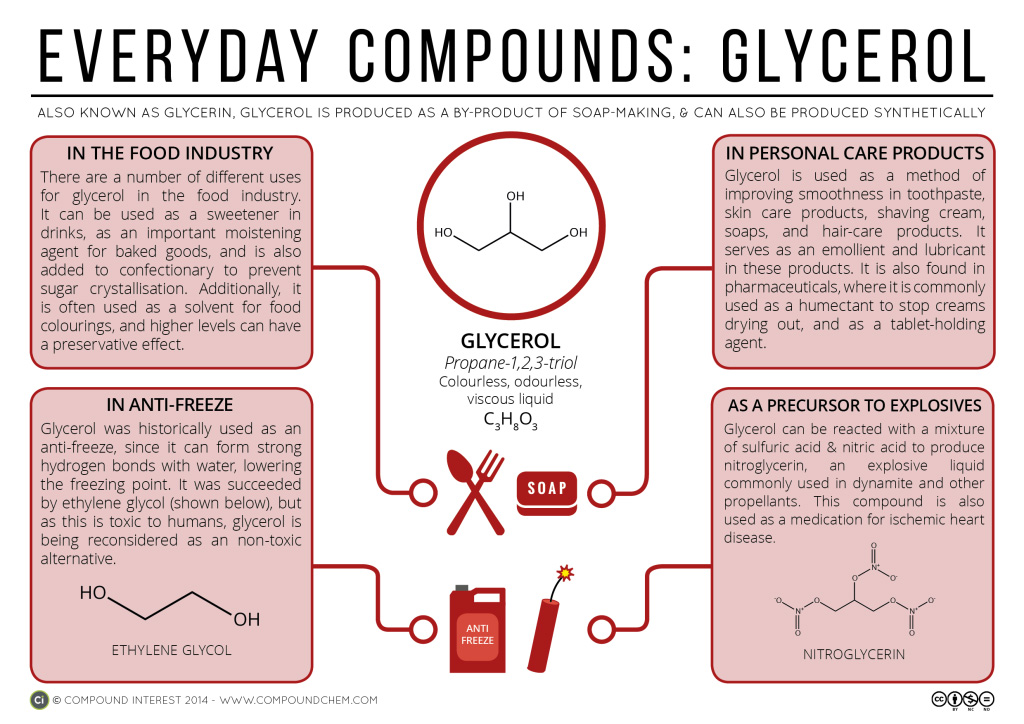
Spotlight on Everyday Chemistry: Glycerol
The physical properties of compounds greatly impact their everyday uses. Infographic 23.2a. highlights some key uses of glycerol (1,2,3-propanetriol).
Attribution & References
Except where otherwise noted, this page is written and adapted by David Wegman and Samantha Sullivan Sauer from:
- “14.3: Physical Properties of Alcohols” & “14.6: Glycols and Glycerol” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
References cited in-text
Glucose. (2023, December 19). In Wikipedia.
National Center for Biotechnology Information (2024a). PubChem Compound Summary for CID 174, Ethylene Glycol. Retrieved January 10, 2024.
National Center for Biotechnology Information (2024b). PubChem Compound Summary for CID 1031, 1-Propanol. Retrieved January 10, 2024.
National Center for Biotechnology Information (2024c). PubChem Compound Summary for CID 8103, 1-Hexanol. Retrieved January 10, 2024.

