26.3 Heterocyclic Nitrogen Compounds
Learning Objectives
By the end of this section, you will be able to:
- Understand how a heterocyclic compound differs from other cyclic hydrocarbons.
- Understand what an alkaloid is.
- Compare alkaloids to human health.
Heterocyclic Amines
Looking back at the various cyclic hydrocarbons discussed previously, we see that all the atoms in the rings of these compounds are carbon atoms. In other cyclic compounds, called heterocyclic compounds (Greek heteros, meaning “other”), nitrogen, oxygen, sulfur, or some other atom is incorporated in the ring. Many heterocyclic compounds are important in medicine and biochemistry. Some compose part of the structure of the nucleic acids, which in turn compose the genetic material of cells and direct protein synthesis.
Heterocyclic amines—compounds in which the nitrogen atom occurs as part of a ring—are also common, and each different heterocyclic ring system has its own parent name. The heterocyclic nitrogen atom is always numbered as position 1.
In addition to nitrogen heterocyclic rings, there are heterocyclic rings that contain oxygen and sulfur as well as nitrogen. Infographic 26.3a. shows the variety of heterocyclic rings in organic chemistry.
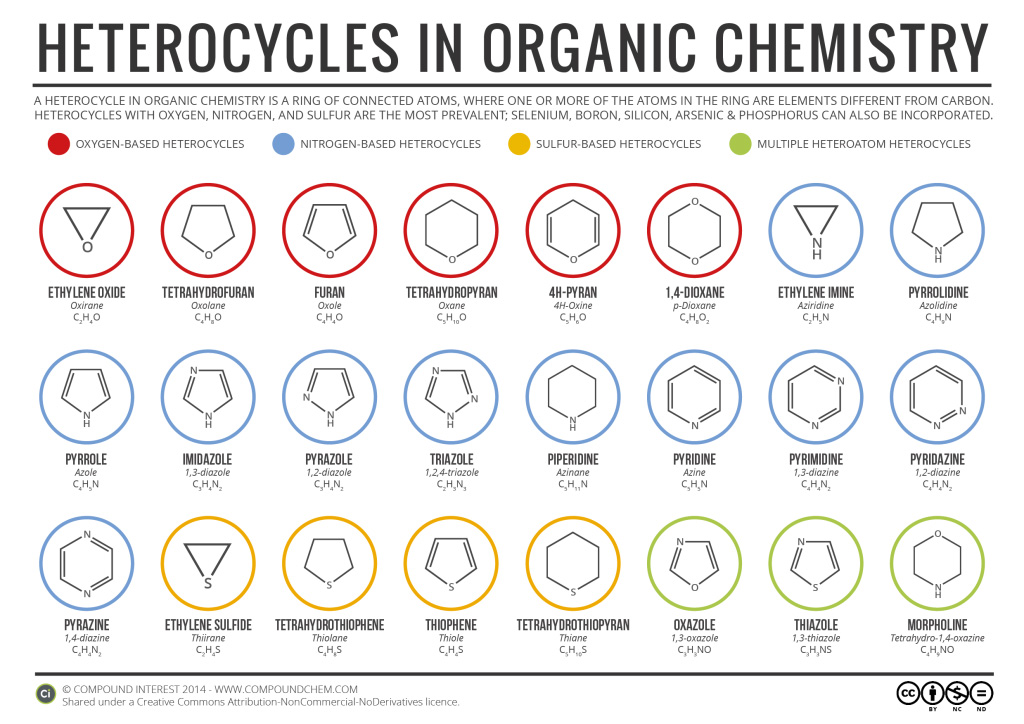
Many heterocyclic amines occur naturally in plants. Like other amines, these compounds are basic. Such a compound is an alkaloid, a name that means “like alkalis.” Many alkaloids are physiologically active, including the familiar drugs caffeine, nicotine, and cocaine.
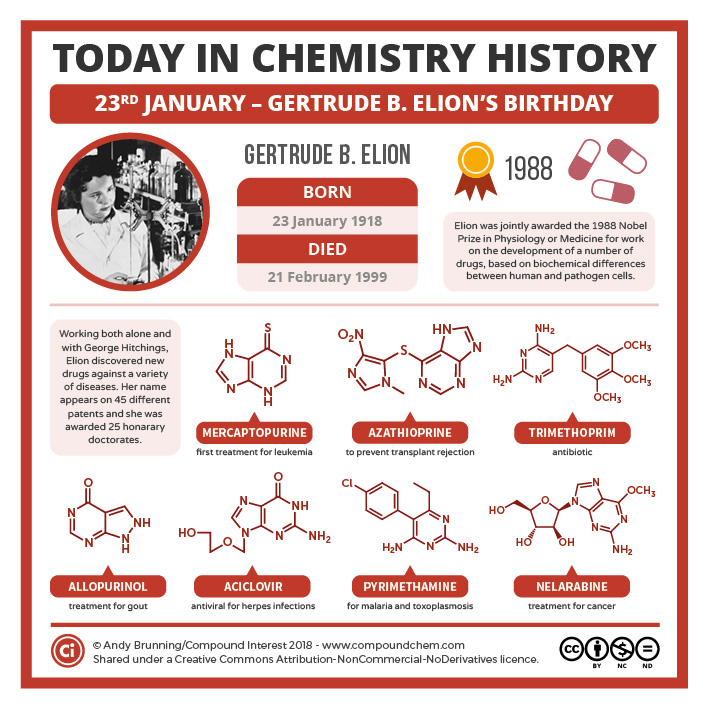
Spotlight on Everday Chemistry: Scientist Gertrude B. Elion
Gertrude B. Elion won the 1988 Nobel Price in Medicine for her work in medications. Many of the medications contain amine structures or heterocyclic rings. Read more in Infographic 26.3b.
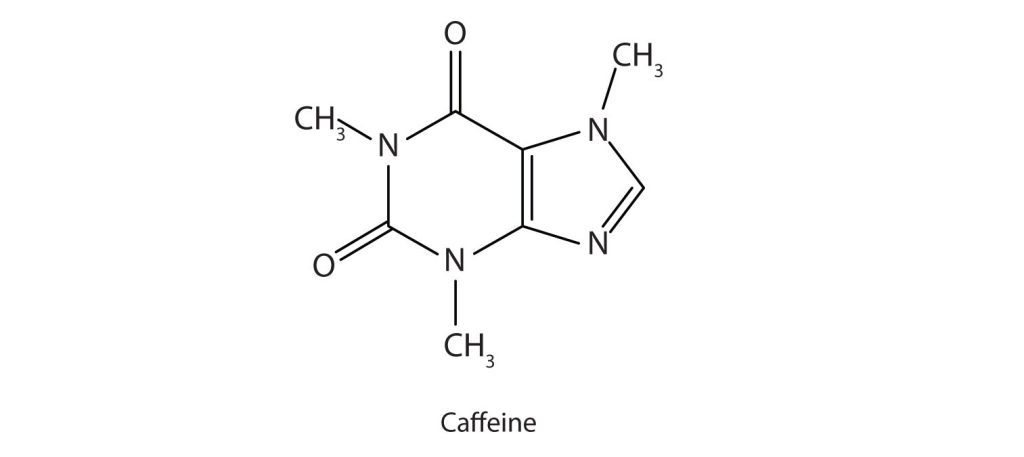
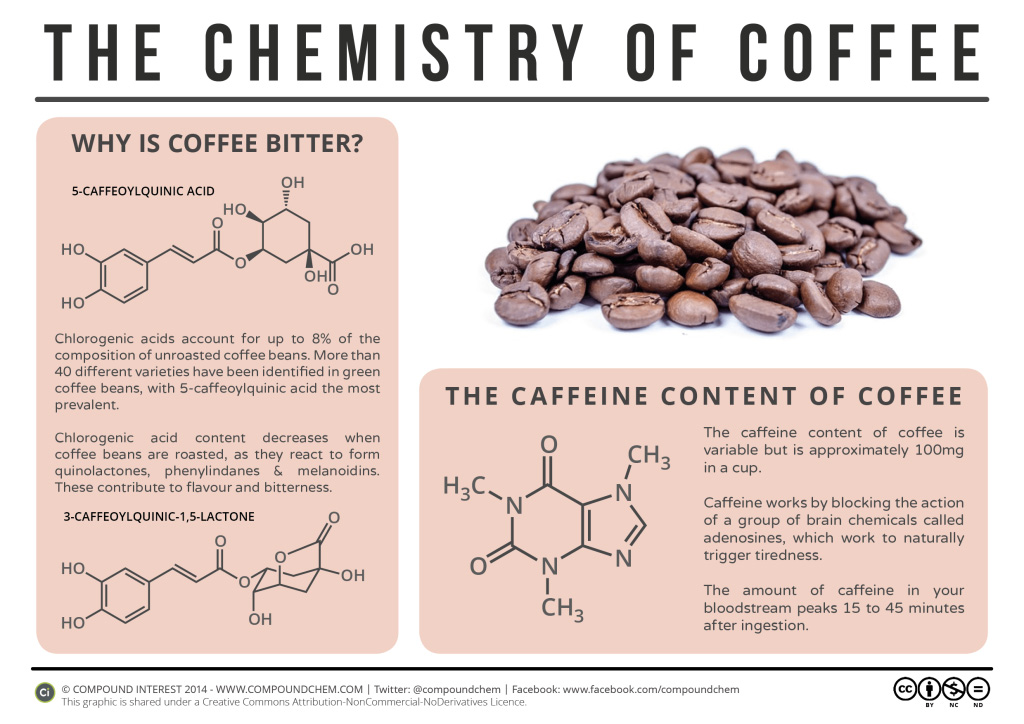
Caffeine (Figure 26.3b.) is a stimulant found in coffee, tea, and some soft drinks. Its mechanism of action is not well understood, but it is thought to block the activity of adenosine, a heterocyclic base that acts as a neurotransmitter, a substance that carries messages across a tiny gap (synapse) from one nerve cell (neuron) to another cell. The effective dose of caffeine is about 200 mg, corresponding to about two cups of strong coffee or tea. Infographic 26.3c. gives more details about coffee including why some brews are bitter.
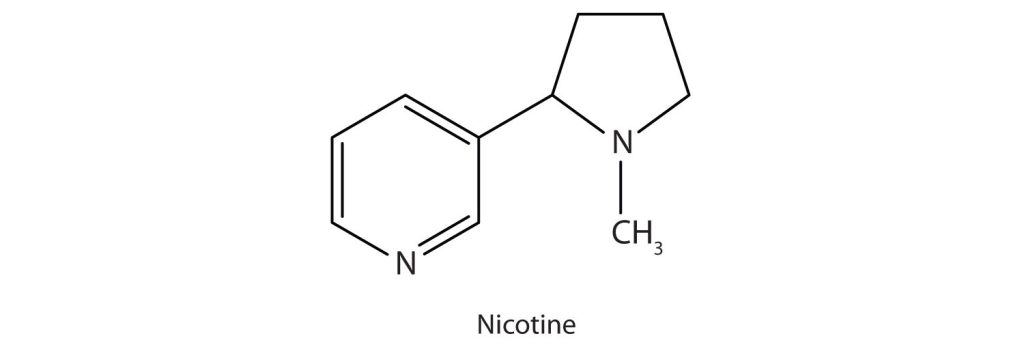
Nicotine (Figure 26.3c.) acts as a stimulant by a different mechanism; it probably mimics the action of the neurotransmitter acetylcholine. People ingest this drug by smoking or chewing tobacco. Its stimulant effect seems transient, as this initial response is followed by depression. Nicotine is highly toxic to animals. It is especially deadly when injected; the lethal dose for a human is estimated to be about 50 mg. Nicotine has also been used in agriculture as a contact insecticide.
Cocaine (Figure 26.3d.) acts as a stimulant by preventing nerve cells from taking up dopamine, another neurotransmitter, from the synapse. High levels of dopamine are therefore available to stimulate the pleasure centers of the brain. The enhancement of dopamine action is thought to be responsible for cocaine’s “high” and its addictive properties. After the binge, dopamine is depleted in less than an hour. This leaves the user in a pleasureless state and (often) craving more cocaine.
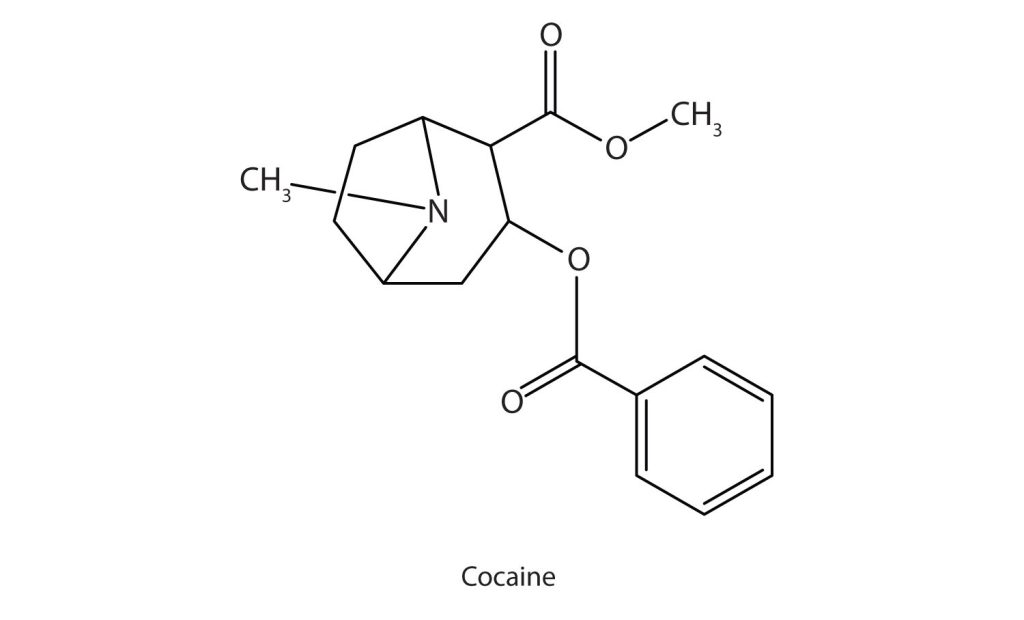
Cocaine is used as the salt cocaine hydrochloride and in the form of broken lumps of the free (unneutralized) base (Figure 26.3e.), which is called crack cocaine.
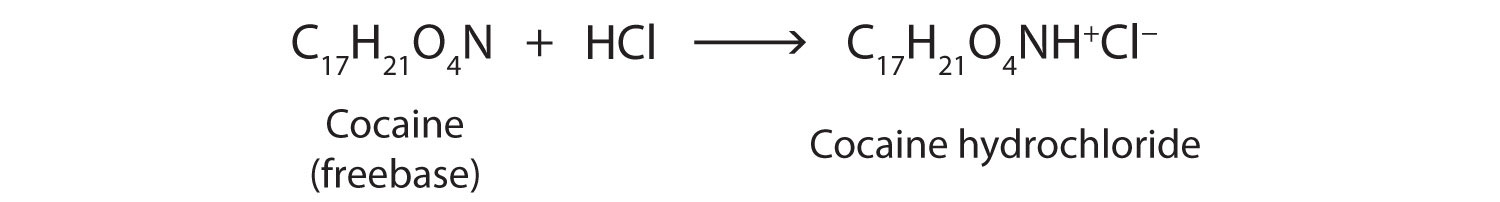
Because it is soluble in water, cocaine hydrochloride is readily absorbed through the watery mucous membranes of the nose when it is snorted. Crack cocaine is more volatile than cocaine hydrochloride. It vaporizes at the temperature of a burning cigarette. When smoked, cocaine reaches the brain in 15 s.
Spotlight on Everyday Chemistry: The Chemistry of Chocolate
Chocolate also has amine and heterocyclic compounds in it and one of these leads to toxicity in dogs. See Infographic 26.3d for more information.

Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey and Samantha Sullivan Sauer from
- “16.2: Naming and Drawing Amines” & “16.4: Heterocyclic Nitrogen Compounds” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0
cyclic or ring shaped compounds containing carbon and other elements such as oxygen, nitrogen or sulfur.
a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom.

