23.5 Phenols
Learning Objectives
By the end of this section, you will be able to:
- Describe the structure and uses of some phenols
- Name phenols that contain 1 or more substituents according to the IUPAC
Structure of Phenols
Compounds in which an OH group is attached directly to an aromatic ring are designated ArOH (where Ar stands for aromatic) and called phenols. Phenols differ from alcohols in that they are slightly acidic in water. They react with aqueous sodium hydroxide (NaOH) to form salts.
ArOH(aq) + NaOH(aq) → ArONa(aq) + H2O
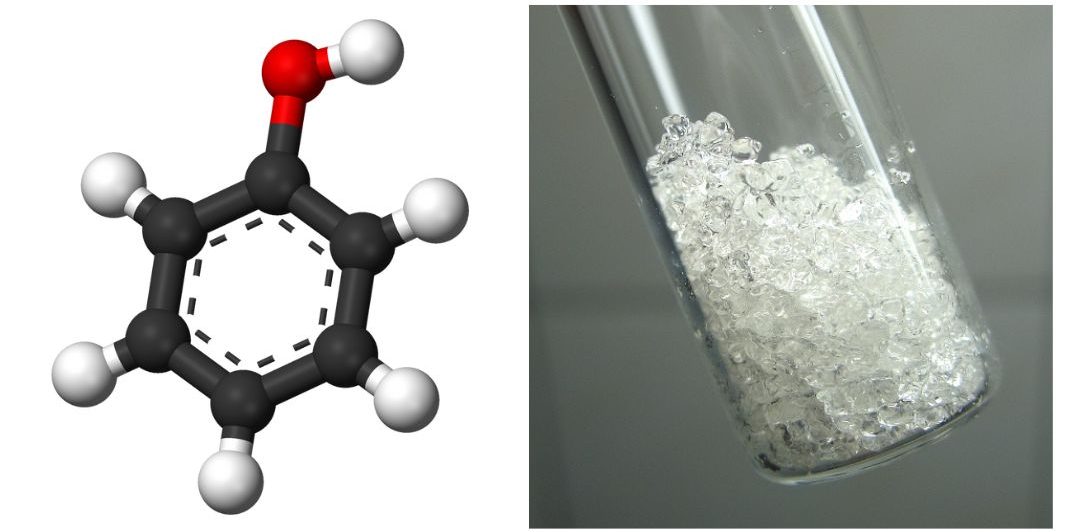
The parent compound, C6H5OH, is itself called phenol. (An old name, emphasizing its slight acidity, was carbolic acid.) Phenol is a white crystalline compound that has a distinctive (“hospital smell”) odour (Figure 23.5a.).
Naming Phenols
In the International Union of Pure and Applied Chemistry (IUPAC) system, the rules for naming phenols are similar to naming substituted aromatics.
- When naming phenols, the parent name is phenol. This accounts for the benzene ring and the hydroxyl attached to it.
- The carbon atom bearing the OH group is designated C1, but the 1 is not used in the name.
- The location of substituents is then determined using the shortest path. The location of all substituents (even if only one is present) must be shown. The name is then determined by indicating the location and identity of the substituents followed by the word phenol.
Example 23.5a
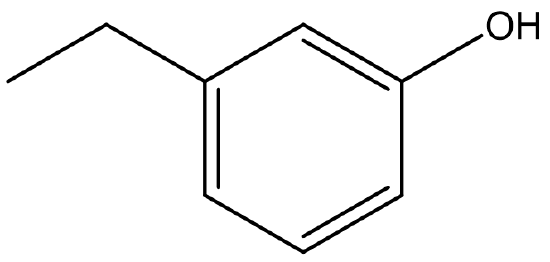
Name the structure in Figure 23.5b.
Solution:
According to the naming rules, the name of the molecule is 3-ethylphenol or meta-ethylphenol.
Example 23.5b
Name each compound.
Solution:
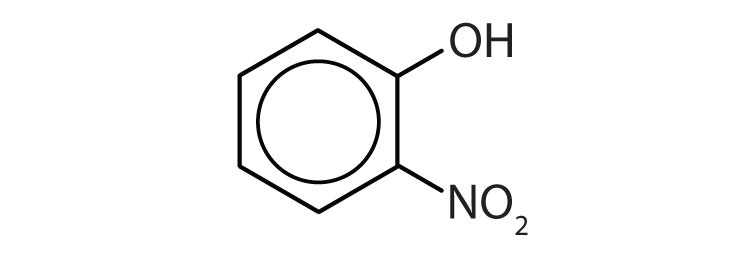
- The parent chain is phenol and C1 would represent the carbon with the OH attached. There is one substituent present on the adjacent carbon, so the parent chain would be numbered clockwise to represent the shortest path. The substituent, which is identified as a nitro group, is on C2. Therefore, the name of the molecule is 2-nitrophenol (or o-nitrophenol).
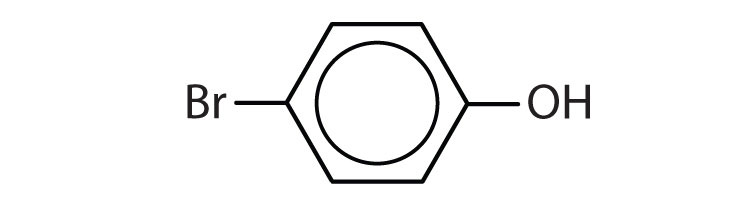
- The parent chain is phenol and C1 would represent the carbon with the OH attached. There is one substituent present on the carbon opposite C1. In this position, the same number is obtained regardless of the chosen path. Therefore, the bromo substituent, is on C4. This indicates that the name of the molecule is 4-bromophenol (or p-bromophenol).
Example source: CHEM 1152: Survey of Chemistry II (GSU – Dr. Osborne) , CC BY-NC-SA 4.0
Exercise 23.5a
Exercise source: CHEM 1152: Survey of Chemistry II (GSU – Dr. Osborne) , CC BY-NC-SA 4.0
Spotlight on Everyday Chemistry: Phenols and Us

Phenols are widely used as antiseptics (substances that kill microorganisms on living tissue) and as disinfectants (substances intended to kill microorganisms on inanimate objects such as furniture or floors). The first widely used antiseptic was phenol (Figure 23.5c.). Joseph Lister used it for antiseptic surgery in 1867. Phenol is toxic to humans, however, and can cause severe burns when applied to the skin. In the bloodstream, it is a systemic poison—that is, one that is carried to and affects all parts of the body. Its severe side effects led to searches for safer antiseptics, a number of which have been found.
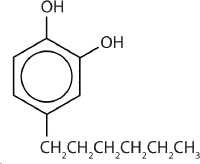
One safer phenolic antiseptic is 4-hexylresorcinol (4-hexyl-1,3-dihydroxybenzene; resorcinol is the common name for 1,3-dihydroxybenzene, and 4-hexylresorcinol has a hexyl group on the fourth carbon atom of the resorcinol ring) (Figure 23.5d.). It is much more powerful than phenol as a germicide and has fewer undesirable side effects. Indeed, it is safe enough to be used as the active ingredient in some mouthwashes and throat lozenges.
Indigenous Perspectives: Maple Syrup
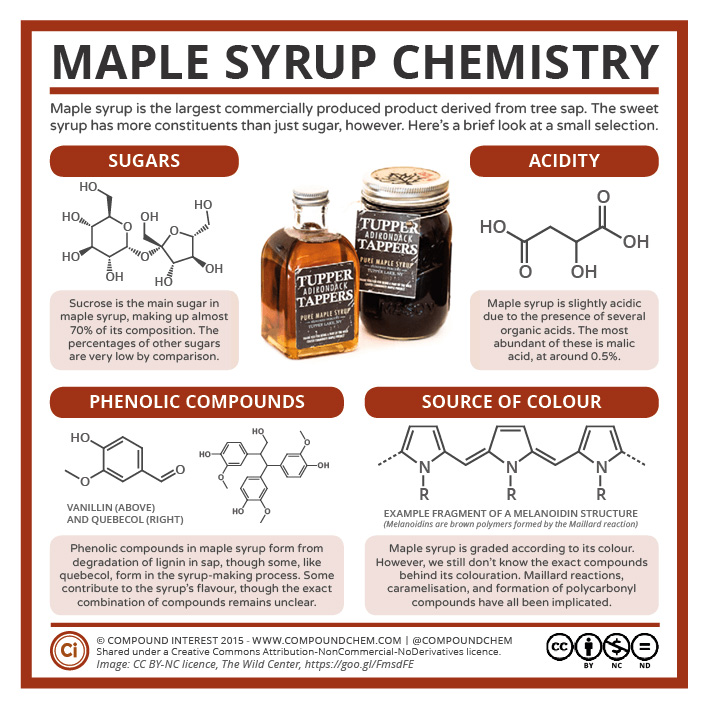
Long before Turtle Island was colonized, Indigenous Peoples of the First Nations used sap from maple trees to produce a sweet syrup or “sweet water” which could also be made into solid blocks. Maple syrup is considered to have healing and nourishing powers. Sweet water is used in ceremonies and for cooking as well. Early colonizers survived due to the gifts of maple products from the local First Nations Peoples. Infographic 23.5a. highlights some of the organic compounds found in maple syrup. Phenol based compounds are key to the flavour of the syrup (Seto, 2021; Wabanaki, n.d.).
Attribution & References
Except where otherwise noted, this page is written and adapted by David Wegman and Samantha Sullivan Sauer from
- “14.7: Phenols” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- Naming phenols is adapted from “3.3: Phenols” In CHEM 1152: Survey of Chemistry II (GSU – Dr. Osborne) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts.
References cited in-text
Seto, C. (2021, July 28). Sweet water. Northern Ontario Travel: The Official Magazine.
Wabanaki. (n.d.). Our story | Indigenous, Female Owned Maple Syrup.
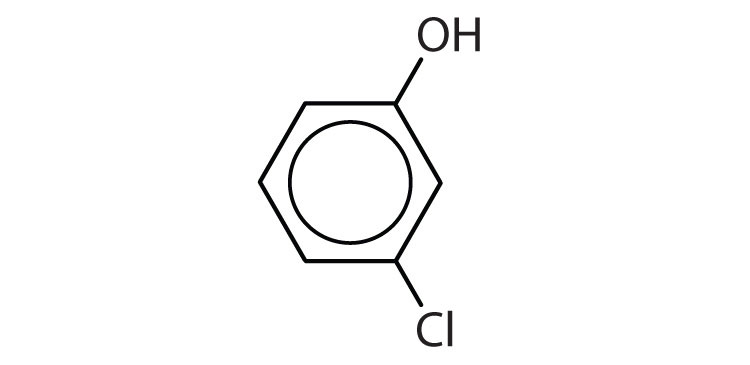
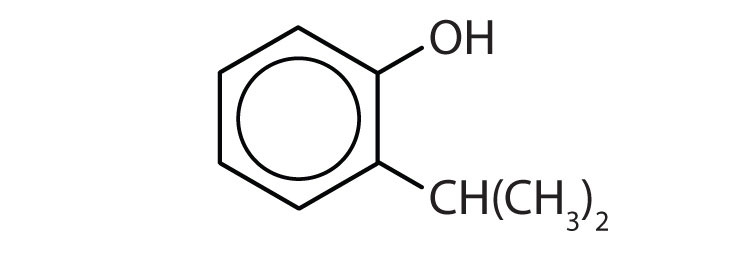
- 1) m-chlorophenol (or 3-chlorophenol 2) o-isopropylphenol (or 2-isopropylphenol) ↵
Compounds in which an OH group is attached directly to an aromatic ring are designated ArOH (where Ar stands for aromatic)