20.2 Alkane Formulas
Learning Objectives
By the end of this section, you will be able to:
- Write molecular formulas for alkanes
- Draw structural formulas of the first 10 alkanes
- Write condensed structural formulas for alkanes given complete structural formulas.
- Draw line structures given condensed or structural formulas.
Types of Formulas to Represent Hydrocarbons
We use several kinds of formulas to describe organic compounds. A molecular formula shows only the kinds and numbers of atoms in a molecule. The general formula for alkanes: CnH2n+ 2. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms. Table 20.2a. shows the molecular formulas of the first 10 straight-chain alkanes.
Table source: “12.2: Structures and Names of Alkanes” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
For example in Table 20.2a. above, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesn’t distinguish between butane and isobutane. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. Thus, structural formulas identify the specific isomers (learned in the next section) by showing the order of attachment of the various atoms.
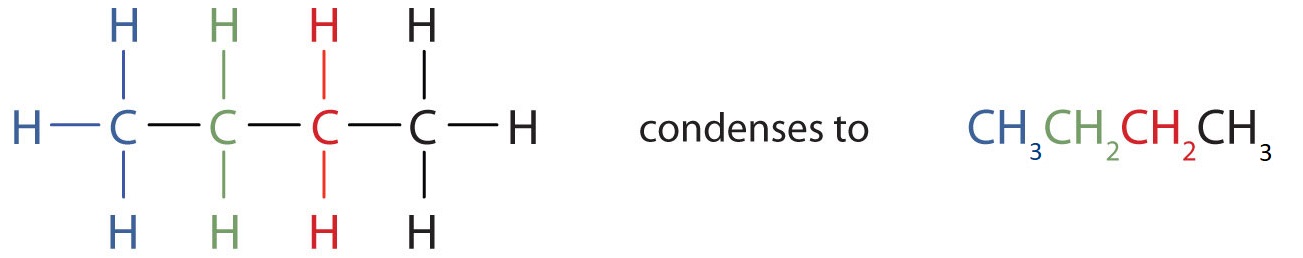
Unfortunately, structural formulas are difficult to type/write and take up a lot of space. Chemists often use condensed structural formulas to alleviate these problems. The condensed structural formulas for the first 10 alkanes are demonstrated in Table 20.2a. Parentheses in condensed structural formulas indicate that the enclosed grouping of atoms is attached to the adjacent carbon atom. The condensed formulas show hydrogen atoms right next to the carbon atoms to which they are attached, as illustrated in Figure 20.2a. for butane:
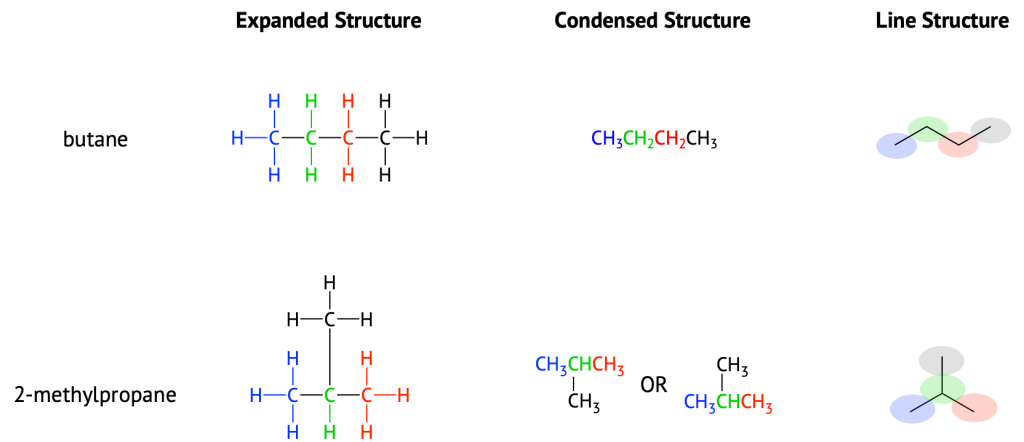
The ultimate condensed formula is a line structure formula. In this type of structure, carbon atoms are not symbolized with a C, but represented by each end of a line or bend in a line. Hydrogen atoms are not drawn if they are attached to a carbon. Other atoms besides carbon and hydrogen are represented by their elemental symbols. For example in Figure 20.2b., we can represent pentane (CH3CH2CH2CH2CH3) and isopentane [(CH3)2CHCH2CH3] as follows:

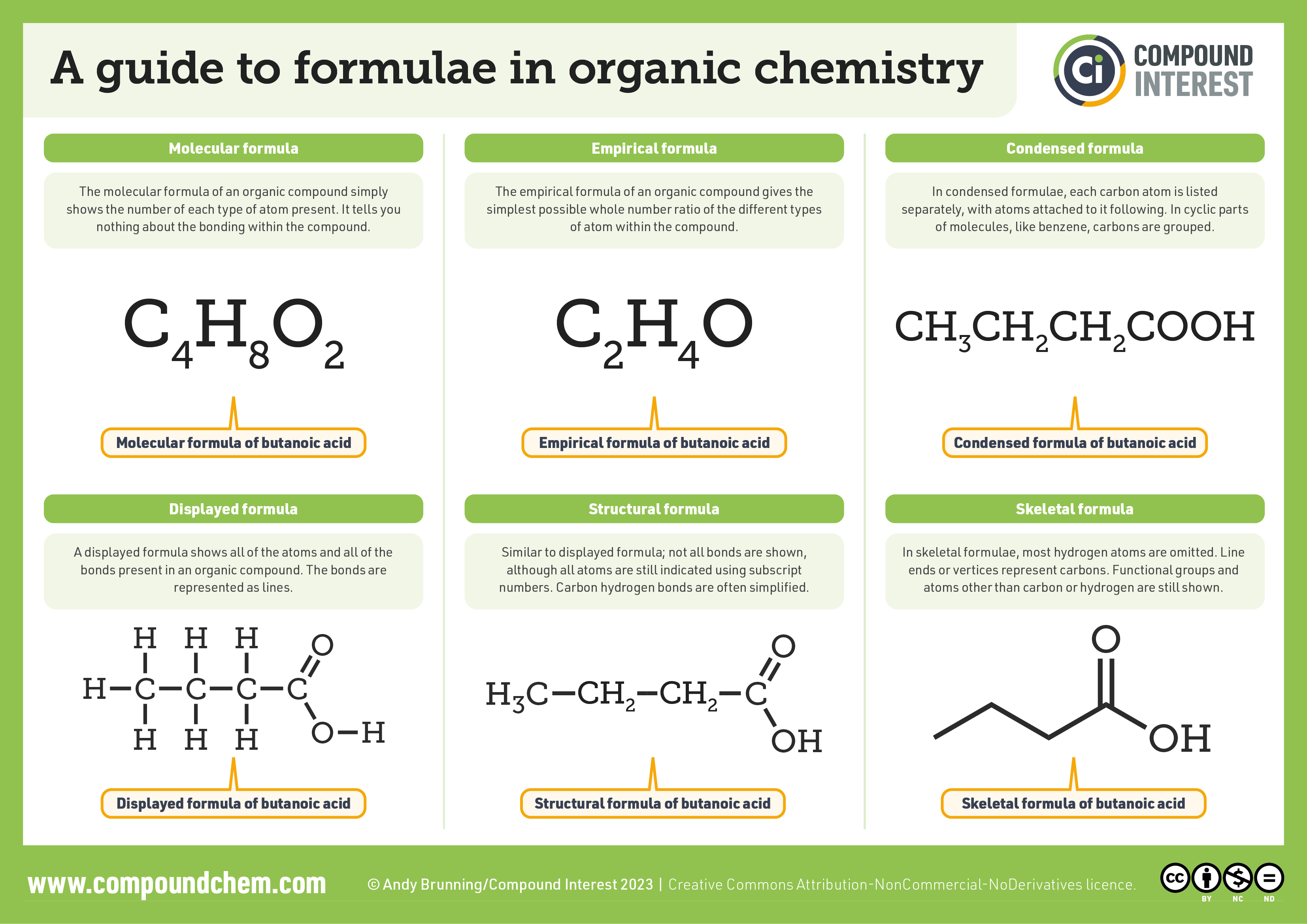
For a summary chart of the formulae used in organic chemistry, infographic 20.2a. demonstrates the different ways to represent organic compounds. Molecular and empirical formulas are explained as the simplest followed by condensed, structural and skeletal (or line) formulae.
Example 20.2a
Draw the line structures for these two molecules:
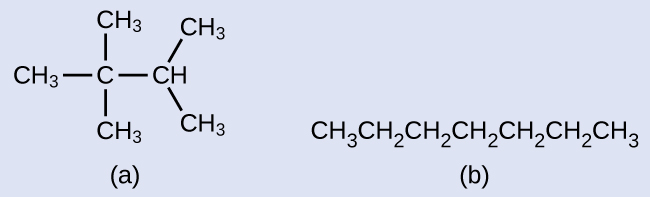
Solution
Each carbon atom is converted into the end of a line or the place where lines intersect. All hydrogen atoms attached to the carbon atoms are left out of the structure (although we still need to recognize they are there):
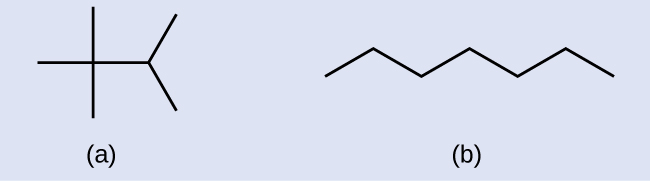
Example & image source: General Chemistry 1 & 2 , CC BY 4.0.
Exercise 20.2a
Draw the line structures for these two molecules:
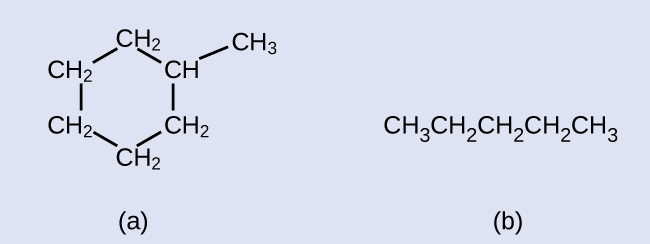
Check your answer[1]
Example & image source: General Chemistry 1 & 2 , CC BY 4.0.
Example 20.2b
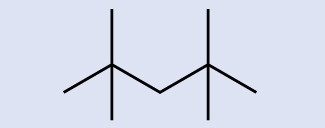
Identify the molecular formula of the molecule represented here:
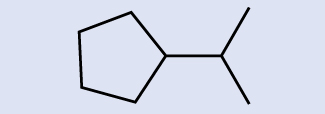
Solution
There are eight places where lines intersect or end, meaning that there are eight carbon atoms in the molecule. Since we know that carbon atoms tend to make four bonds, each carbon atom will have the number of hydrogen atoms that are required for four bonds. This compound contains 16 hydrogen atoms for a molecular formula of C8H16.
Location of the hydrogen atoms:
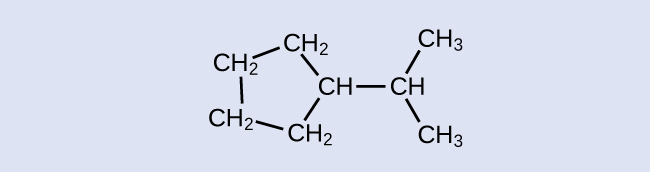
Example & image source: General Chemistry 1 & 2 , CC BY 4.0.
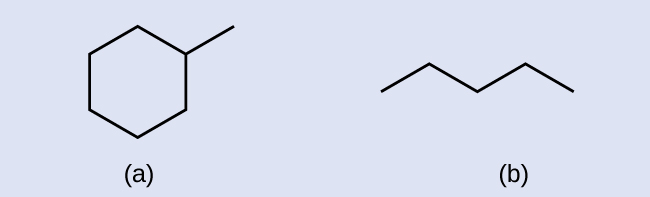
Exercise 20.2b
Check your Answer[2]
Example & image source: General Chemistry 1 & 2 , CC BY 4.0.
Exercise 20.2c
Identifying carbons in a structure
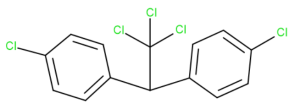
By looking at the chemical structure of DDT (Dichlorodiphenyltrichloroethane), click on the location of each carbon within the DDT structure.
Exercise 20.2c (Text version)
Identify the location of each carbon atom in the DDT structure. DDT is dichlorodiphenyltrichloroethane. It contains two aromatic rings connected by one carbon of an ethane structure. The molecular formula is C14H9Cl5. (Structure is shown in as a line structure.)
Check Your Answer:[3]
Activity source: “Identifying carbons in line structure” by Samantha Sullivan Sauer, licensed under CC BY-NC 4.0
Links to Enhanced Learning
- For an interactive practice link to the interactive activity on Hydrocarbons [New tab] to determine the number of hydrogens in an organic compound from Jessica Anderson.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from
- “12.2: Structures and Names of Alkanes“, “12.4: Condensed Structural and Line-Angle Formulas” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
- Individual pages have been remixed into this version, including
- Examples 1 & 2 are adapted from “18.1 Hydrocarbons” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “12.4: Drawing Organic Structures” by Lisa Sharpe Elles, In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0. / A derivative of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0. which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
The formula that shows only the kinds and numbers of atoms in a molecule
The formula that shows the elements and their arrangements within the molecular structure
The formula where carbon atoms are not symbolized with a C, but represented by each end of a line or bend in a line. Hydrogen atoms are not drawn if they are attached to a carbon

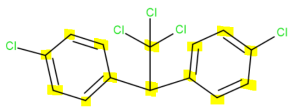 There are 14 carbon atoms. Six in each aromatic ring, one that connects the rings together and one that branches off the middle carbon.
There are 14 carbon atoms. Six in each aromatic ring, one that connects the rings together and one that branches off the middle carbon. 