Chapter 26: Amides and Amines
Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry
by Gregory Anderson; Jen Booth; Caryn Fahey; Adrienne Richards; Samantha Sullivan Sauer; and David Wegman
Chapter Contents
- 26.1 Amines – Structures and Naming
- 26.2 Amines – Physical Properties
- 26.3 Heterocyclic Nitrogen Compounds
- 26.4 Basicity of Amines
- 26.5 Amides – Structures, Properties and Naming
- 26.6 Chemical Properties of Amines and Amides
- Chapter 26 – Summary
- Chapter 26 – Review
- Chapter 26 – Infographic descriptions
Except where otherwise noted, this OER is licensed under CC BY-NC-SA 4.0
Please visit the web version of Organic and Biochemistry Supplement to Enhanced Introductory College Chemistry to access the complete book, interactive activities and ancillary resources.
In this chapter, you will learn about:
- Amines: what are they? What is their chemical structure? What are the physical and chemical properties of these nitrogen containing compounds?
- Amides: what are they? What is their chemical structure? What are the physical and chemical properties of amides?
To better support your learning, you should be familiar with the following concepts before starting this chapter:
- Alkanes, Alkenes, and Alkynes (Chapter 20: Alkanes and Akyl Halides and Chapter 22: Alkenes, Alkynes and Aromatics)
- Alcohols and Ethers (Chapter 23: Alcohols and Ethers)
- Aldehydes and Ketones (Chapter 24: Aldehydes and Ketones)
- Carboxylic Acids and Esters (Chapter 25: Carboxylic Acids and Esters)
- Functional Groups (Chapter 19.5: Families of Organic Molecules)

By the end of this chapter, we will have seen all the common functional groups. Of those groups, carbonyl compounds and amines are the most abundant and have the richest chemistry. In addition to proteins and nucleic acids, the majority of pharmaceutical agents contain amine functional groups, and many of the common coenzymes necessary for biological catalysis are amines.
Amines are organic derivatives of ammonia in the same way that alcohols and ethers are organic derivatives of water. Like ammonia, amines contain a nitrogen atom with a lone pair of electrons, making amines both basic and nucleophilic.
Amines occur widely in all living organisms. Trimethylamine, for instance, occurs in animal tissues and is partially responsible for the distinctive odour of fish; nicotine is found in tobacco; and cocaine is a stimulant found in the leaves of the South American coca bush. In addition, amino acids are the building blocks from which all proteins are made, and cyclic amine bases are constituents of nucleic acids.
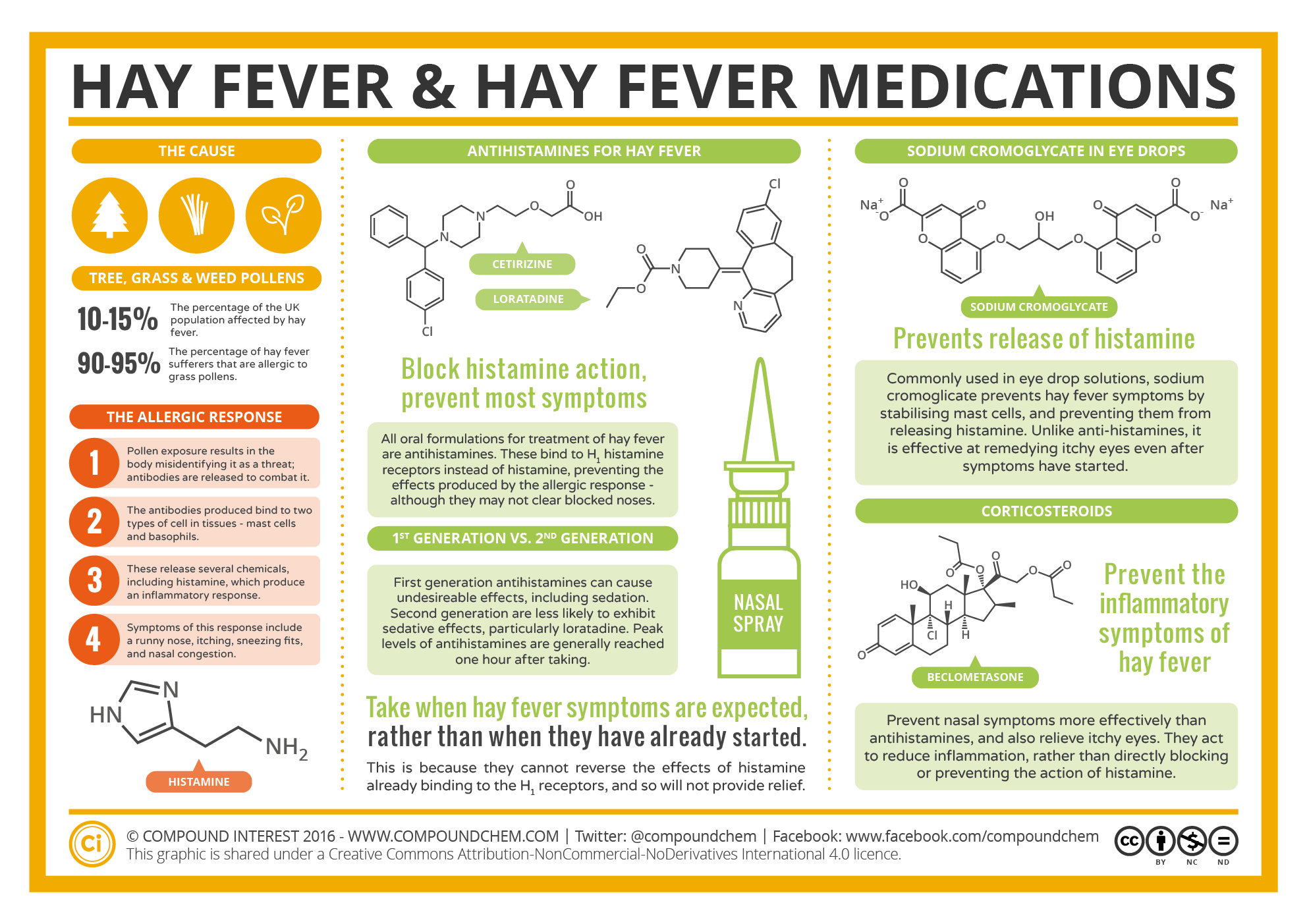
Spotlight on Everyday Chemistry: Hay Fever
Many everyday chemicals and medications rely on the amine and amide functional groups. Infographic 26.0a. shows the applications of amines and amides to hay fever medication.
Closely related to carboxylic acids and nitriles are the carboxylic acid derivatives, compounds in which an acyl group is bonded to an electronegative atom or substituent that can act as a leaving group in the nucleophilic acyl substitution reaction.
Many kinds of acid derivatives are known, one of those being amides. Amides are common in both laboratory and biological chemistry. Amides are molecules that contain nitrogen atoms connected to the carbon atom of a carbonyl group.
Watch Amines: Crash Course Organic Chemistry #46 – Youtube (12 min)
Video Source: Crash Course Chemistry. (2022, February 16). Amines: Crash Course organic chemistry #46 [Video]. YouTube.
The table below outlines all organic compounds discussed thus far including their functional groups, formulas and names.
Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey from:
- “20.4 Amines and Amides” In Chemistry 2e (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley, R. Robinson & R. William, licensed under CC BY 4.0. Access for free at Chemistry 2e (Open Stax)
- “Carboxylic Acid Derivatives: Why This Chapter?” In Organic Chemistry (OpenStax) by John McMurray, licensed under CC BY-NC-SA 4.0. Access for free at Organic Chemistry (OpenStax)
- “15.0: Prelude to Organic Acids and Bases and Some of Their Derivatives” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
organic molecule in which a nitrogen atom is bonded to one or more alkyl group
organic molecule that features a nitrogen atom connected to the carbon atom in a carbonyl group

