28.5 Vitamins
Learning Objectives
By the end of this section, you will be able to:
- Explain why vitamins are necessary in the diet
- Explain why some vitamins are water soluble and some are lipid soluble.
- Describe the functional role, intake recommendations and sources of vitamins.
- Explain the role of vitamins as antioxidants and as coenzymes.
Vitamins are essential to human health and can be obtained in our diet from different types of food.
The Vitamins: Vital, but Not All are Amines
In 1747, the Scottish surgeon James Lind discovered that citrus foods helped prevent scurvy, a particularly deadly disease in which collagen is not properly formed, causing poor wound healing, bleeding of the gums, severe pain, and death. In 1753, Lind published his Treatise on the Scurvy, which recommended using lemons and limes to avoid scurvy, which was adopted by the British Royal Navy. This led to the nickname limey for British sailors.
In East Asia, where polished white rice was the common staple food of the middle class, beriberi resulting from lack of vitamin B1 was endemic. In 1884, Takaki Kanehiro, a British-trained medical doctor of the Imperial Japanese Navy, observed that beriberi was endemic among low-ranking crew who often ate nothing but rice, but not among officers who consumed a Western-style diet. This convinced Takaki and the Japanese Navy that diet was the cause of beriberi, but they mistakenly believed that sufficient amounts of protein prevented it. That diseases could result from some dietary deficiencies was further investigated by Christiaan Eijkman, who in 1897 discovered that feeding unpolished rice instead of the polished variety to chickens helped to prevent beriberi in the chickens. The following year, Frederick Hopkins postulated that some foods contained “accessory factors” — in addition to proteins, carbohydrates, fats etc. — that are necessary for the functions of the human body. Hopkins and Eijkman were awarded the Nobel Prize for Physiology or Medicine in 1929 for their discoveries.
In 1910, the first vitamin complex was isolated by Japanese scientist Umetaro Suzuki, who succeeded in extracting a water-soluble complex of micronutrients from rice bran and named it aberic acid (later Orizanin). He published this discovery in a Japanese scientific journal. When the article was translated into German, the translation failed to state that it was a newly discovered nutrient, a claim made in the original Japanese article, and hence his discovery failed to gain publicity. In 1912 Polish-born biochemist Casimir Funk, working in London, isolated the same complex of micronutrients and proposed the complex be named “vitamine”. It was later to be known as vitamin B3 (niacin), though he described it as “anti-beri-beri-factor” (which would today be called thiamine or vitamin B1). Funk proposed the hypothesis that other diseases, such as rickets, pellagra, coeliac disease, and scurvy could also be cured by vitamins.
Max Nierenstein a friend and reader of Biochemistry at Bristol University reportedly suggested the “vitamine” name (from “vital amine”). The name soon became synonymous with Hopkins’ “accessory factors”, and, by the time it was shown that not all vitamins are amines, the word was already ubiquitous. In 1920, Jack Cecil Drummond proposed that the final “e” be dropped to deemphasize the “amine” reference, after researchers began to suspect that not all “vitamines” (in particular, vitamin A) have an amine component (Figure 28.5a.).

Vitamins are organic compounds found in foods and are a necessary part of the biochemical reactions in the body (Figure 28.5b.). They are involved in a number of processes, including mineral and bone metabolism, and cell and tissue growth, and they act as cofactors for energy metabolism.
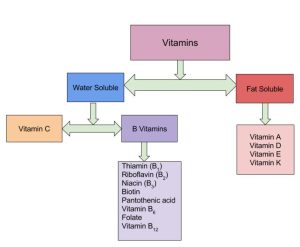
You get most of your vitamins through your diet, although some can be formed from the precursors absorbed during digestion. For example, the body synthesizes vitamin A from the β-carotene in orange vegetables like carrots and sweet potatoes. Vitamins are either fat-soluble or water-soluble. Fat-soluble vitamins A, D, E, and K, are absorbed through the intestinal tract with lipids in chylomicrons. Vitamin D is also synthesized in the skin through exposure to sunlight. Because they are carried in lipids, fat-soluble vitamins can accumulate in the lipids stored in the body. If excess vitamins are retained in the lipid stores in the body, hypervitaminosis can result.
Water-soluble vitamins, including the eight B vitamins and vitamin C, are absorbed with water in the gastrointestinal tract. These vitamins move easily through bodily fluids, which are water based, so they are not stored in the body. Excess water-soluble vitamins are excreted in the urine. Therefore, hypervitaminosis of water-soluble vitamins rarely occurs, except with an excess of vitamin supplements. The B vitamins play the largest role of any vitamins in metabolism (Table 28.5a. and Table 28.5b.).
All fat-soluble vitamins contain a high proportion of hydrocarbon structural components. There are one or two oxygen atoms present, but the compounds as a whole are nonpolar. In contrast, water-soluble vitamins contain large numbers of electronegative oxygen and nitrogen atoms, which can engage in hydrogen bonding with water. Most water-soluble vitamins act as coenzymes or are required for the synthesis of coenzymes. The fat-soluble vitamins are important for a variety of physiological functions. A coenzyme is an organic molecule that is necessary for an enzyme’s proper functioning. It is one type of cofactor; another type is inorganic ions.
Fat Soluble Vitamins
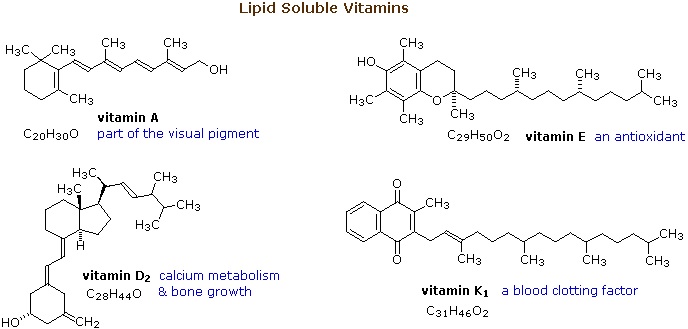
From the structures in Figure 28.5c., it should be clear that these compounds have more than a solubility connection with lipids. Vitamin A is a terpene, and vitamins E and K have long terpene chains attached to an aromatic moiety. The structure of vitamin D can be described as a steroid in which ring B is cut open and the remaining three rings remain unchanged. The precursors of vitamins A and D have been identified as the tetraterpene beta-carotene and the steroid ergosterol, respectively. Table 28.5a. lists the different fat-soluble vitamins and its function.
| Vitamin and alternative name | Sources | Recommended daily allowance | Function | Problems associated with deficiency |
|---|---|---|---|---|
| A
retinal or β-carotene |
Yellow and orange fruits and vegetables, dark green leafy vegetables, eggs, milk, liver | 700–900 µg | Eye and bone development, immune function | Night blindness, epithelial changes, immune system deficiency |
| D
cholecalciferol |
Dairy products, egg yolks; also synthesized in the skin from exposure to sunlight | 5–15 µg | Aids in calcium absorption, promoting bone growth | Rickets, bone pain, muscle weakness, increased risk of death from cardiovascular disease, cognitive impairment, asthma in children, cancer |
| E
tocopherols |
Seeds, nuts, vegetable oils, avocados, wheat germ | 15 mg | Antioxidant | Anemia |
| K
phylloquinone |
Dark green leafy vegetables, broccoli, Brussels sprouts, cabbage | 90–120 µg | Blood clotting, bone health | Hemorrhagic disease of newborn in infants; uncommon in adults |
Links to Enhanced Learning
More detailed information on the different fat-soluble vitamins can be found at 9.2: Fat-Soluble Vitamins – Medicine LibreTexts.
Water Soluble Vitamins
All water-soluble vitamins (Table 28.5b.) play a different kind of role in energy metabolism; they are required as functional parts of enzymes involved in energy release and storage. Vitamins and minerals that make up part of enzymes are referred to as coenzymes and cofactors, respectively. Coenzymes and cofactors are required by enzymes to catalyze a specific reaction. They assist in converting a substrate to an end-product. Coenzymes and cofactors are essential in catabolic pathways and play a role in many anabolic pathways too. In addition to being essential for metabolism, many vitamins and minerals are required for blood renewal and function. At insufficient levels in the diet these vitamins and minerals impair the health of blood and consequently the delivery of nutrients in and wastes out, amongst its many other functions.
| Vitamin and alternative name | Sources | Recommended daily allowance | Function | Problems associated with deficiency |
|---|---|---|---|---|
| B1
thiamine |
Whole grains, enriched bread and cereals, milk, meat | 1.1–1.2 mg | Carbohydrate metabolism | Beriberi, Wernicke-Korsikoff syndrome |
| B2
riboflavin |
Brewer’s yeast, almonds, milk, organ meats, legumes, enriched breads and cereals, broccoli, asparagus | 1.1–1.3 mg | Synthesis of FAD for metabolism, production of red blood cells | Fatigue, slowed growth, digestive problems, light sensitivity, epithelial problems like cracks in the corners of the mouth |
| B3
niacin |
Meat, fish, poultry, enriched breads and cereals, peanuts | 14–16 mg | Synthesis of NAD, nerve function, cholesterol production | Cracked, scaly skin; dementia; diarrhea; also known as pellagra |
| B5
pantothenic acid |
Meat, poultry, potatoes, oats, enriched breads and cereals, tomatoes | 5 mg | Synthesis of coenzyme A in fatty acid metabolism | Rare: symptoms may include fatigue, insomnia, depression, irritability |
| B6
pyridoxine |
Potatoes, bananas, beans, seeds, nuts, meat, poultry, fish, eggs, dark green leafy vegetables, soy, organ meats | 1.3–1.5 mg | Sodium and potassium balance, red blood cell synthesis, protein metabolism | Confusion, irritability, depression, mouth and tongue sores |
| B7
biotin |
Liver, fruits, meats | 30 µg | Cell growth, metabolism of fatty acids, production of blood cells | Rare in developed countries; symptoms include dermatitis, hair loss, loss of muscular coordination |
| B9
folic acid |
Liver, legumes, dark green leafy vegetables, enriched breads and cereals, citrus fruits | 400 µg | DNA/protein synthesis | Poor growth, gingivitis, appetite loss, shortness of breath, gastrointestinal problems, mental deficits |
| B12
cyanocobalamin |
Fish, meat, poultry, dairy products, eggs | 2.4 µg | Fatty acid oxidation, nerve cell function, red blood cell production | Pernicious anemia, leading to nerve cell damage |
| C
ascorbic acid |
Citrus fruits, red berries, peppers, tomatoes, broccoli, dark green leafy vegetables | 75–90 mg | Necessary to produce collagen for formation of connective tissue and teeth, and for wound healing | Dry hair, gingivitis, bleeding gums, dry and scaly skin, slow wound healing, easy bruising, compromised immunity; can lead to scurvy |
Links to Enhanced Learning
Exercise 28.5a
Using Infographic 28.5a., identify the functional groups that make the water-soluble vitamins soluble.
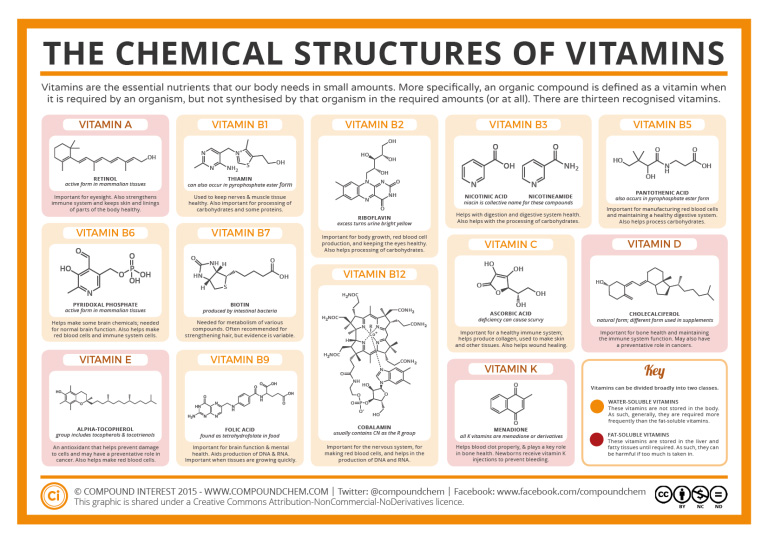
Solution:
| Water soluble vitamin | Functional groups responsible for water solubility |
|---|---|
| B1 | Amine, alcohol, sulfur group |
| B2 | Alcohol, amide, amine |
| B3 | (left) carboxylic acid, amine (right) amide, amine |
| B5 | Alcohol, carboxylic acid, amide |
| B6 | Aldehyde, alcohol, amine, phosphate group |
| B7 | Amide, carboxylic acid, sulfur group |
| B9 | Amide, amine, carboxylic acid |
| B12 | Amide, alcohol, amine, phosphate group |
| C | Ester, alcohol |
Source: Except where otherwise noted, Exercise 28.5a by Samantha Sullivan Sauer is licensed under CC BY-NC 4.0.
Indigenous Perspectives: Inuit Nutrition

Sources of vitamins are dependent on access to specific types of food. In the Arctic, food sources are limited. The Inuit traditional diet has been studied to understand the source of key vitamins available in Arctic conditions (Naqitarvik et al, 2022):
- Vitamin A: obtained from liver of animals such as polar bears. Typically eaten raw.
- Vitamin D: obtained from liver of animals.
- Vitamin C: obtained from liver of animals (raw only), berries, raw fish eggs, raw whale skin (maktaaq/mattak)
- Vitamin K1: obtained from rhubarb
- Omega fatty acids: obtained from seal blubber (uqsuq) and oil
For more details, read this article: Living on the Edge | Chem 13 News Magazine | University of Waterloo (uwaterloo.ca)
Vitamins as Antioxidants
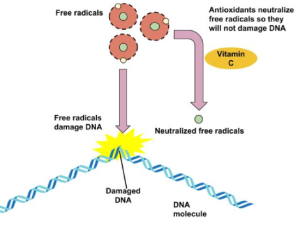
The “big three” vitamin antioxidants are vitamins E, A, and C, although it may be that they are called the “big three” only because they are the most studied. Antioxidants prevent damage from free radicals, which are molecules that are highly reactive because they have unpaired electrons. Free radicals are formed not only through metabolic reactions involving oxygen but also by such environmental factors as radiation and pollution. Free radicals react most commonly with lipoproteins and unsaturated fatty acids in cell membranes, removing an electron from those molecules and thus generating a new free radical. The process becomes a chain reaction that finally leads to the oxidative degradation of the affected compounds. Antioxidants react with free radicals to stop these chain reactions by forming a more stable molecule or, in the case of vitamin E, a free radical that is much less reactive (vitamin E is converted back to its original form through interaction with vitamin C). A simplified diagram on the role of antioxidants with DNA is shown in Figure 28.5e. Here, antioxidants neutralize free radicals to prevent DNA damage. Preventing DNA damage helps maintain our genetic code. Other antioxidants obtained from the diet are given in Table 28.5c.
| Antioxidant | Functions Attributed to Antioxidant Capacity |
|---|---|
| Vitamin A | Protects cellular membranes, prevents glutathione depletion, maintains free radical detoxifying enzyme systems, reduces inflammation |
| Vitamin E | Protects cellular membranes, prevents glutathione depletion |
| Vitamin C | Protects DNA, RNA, proteins, and lipids, aids in regenerating vitamin E |
| Carotenoids | Free radical scavengers |
| Lipoic acid | Free radical scavenger, aids in regeneration of vitamins C and E |
| Phenolic acids | Free radical scavengers, protect cellular membranes |
Table source: Wikipedia, Chemistry for Changing Times (Hill & McCreary), CC BY-NC-SA 4.0.
Effects of Cooking
The USDA has conducted extensive studies on the percentage losses of various nutrients from different food types and cooking methods. Some vitamins may become more “bio-available” – that is, usable by the body – when foods are cooked. Table 28.5d. shows whether various vitamins are susceptible to loss from heat—such as heat from boiling, steaming, frying, etc. The effect of cutting vegetables can be seen from exposure to air and light. Water-soluble vitamins such as B and C dissolve into the water when a vegetable is boiled and are then lost when the water is discarded.
| Vitamin | Soluble in Water | Stable to Air Exposure | Stable to Light Exposure | Stable to Heat Exposure |
|---|---|---|---|---|
| Vitamin A | no | partially | partially | relatively stable |
| Vitamin C | very unstable | yes | no | no |
| Vitamin D | no | no | no | no |
| Vitamin E | no | yes | yes | no |
| Vitamin K | no | no | yes | no |
| Thiamine (B1) | highly | no | ? | > 100 °C |
| Riboflavin (B2) | slightly | no | in solution | no |
| Niacin (B3) | yes | no | no | no |
| Pantothenic Acid (B5) | quite stable | no | no | yes |
| Vitamin B6 | yes | ? | yes | ? |
| Biotin (B7) | somewhat | ? | ? | no |
| Folic Acid (B9) | yes | ? | when dry | at high temp |
| Cobalamin (B12) | yes | ? | yes | no |
Table source: Wikipedia, Chemistry for Changing Times (Hill & McCreary), CC BY-NC-SA 4.0.
Attribution & References
Except where otherwise noted, this page has been adapted by Samantha Sullivan Sauer from
- “17.4: Minerals, Vitamins, and Other Essentials” In Chemistry for Changing Times (Hill & McCreary) by LibreTexts, licensed under CC BY-NC-SA 4.0. Contributors from original source:
- “18.9: Enzyme Cofactors and Vitamins” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
Modifications: combined the two sources, removed mention of minerals and fibre from material
References cited in-text
Naqitarvik, R., Anderson, C. C., & Rayner-Canham, G. (2022, Fall). Living on the edge: Some chemistry of the Inuit diet. Chem 13 News Magazine.

