23.3 Formation of Alcohols
Learning Objectives
By the end of this section, you will be able to:
- Describe how to prepare alcohols from alkenes
- Describe other methods to prepare alcohols
Organic functional groups can be converted into other functional groups through reactions. A map of some of the more common reactions to convert functional groups can be found in Section 19.6 - General Reactions of Carbon in Infographic 19.6a.
Preparation of Methanol and Ethanol
Methanol is prepared by combining hydrogen gas and carbon monoxide at high temperatures and pressures in the presence of a catalyst composed of zinc oxide (ZnO) and chromium oxide (Cr2O3) catalyst (Figure 23.3a.).
Figure 23.3a. Preparation of methanol from carbon monoxide. (Credit: Introduction to Chemistry: GOB (V. 1.0)., CC BY-NC-SA 3.0.)
Methanol is an important solvent and is used as an automotive fuel, either as the pure liquid—as in some racing cars—or as an additive in gasoline. Nearly 2 billion gallons of methanol are produced each year in the United States by the catalytic reduction of carbon monoxide with hydrogen gas.
Ethanol, CH3CH2OH, also called ethyl alcohol, is a particularly important alcohol for human use. Ethanol is the alcohol produced by some species of yeast that is found in wine, beer, and distilled drinks. It is made by the fermentation of sugars or starch from various sources (potatoes, corn, wheat, rice, etc.). It has long been prepared by humans harnessing the metabolic efforts of yeasts in fermenting various sugars (Figure 23.3b.).
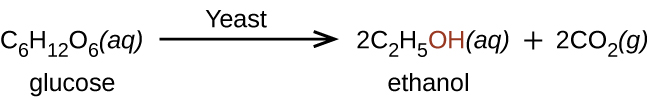
Organic and biochemical equations are frequently written showing only the organic reactants and products. In this way, we focus attention on the organic starting material and product, rather than on balancing complicated equations.
Spotlight on Everyday Chemistry: Physiological Effects of Alcohols
Methanol is quite poisonous to humans. Ingestion of as little as 15 mL of methanol can cause blindness, and 30 mL (1 oz) can cause death. However, the usual fatal dose is 100 to 150 mL. The main reason for methanol’s toxicity is that we have liver enzymes that catalyze its oxidation to formaldehyde (methanal), the simplest member of the aldehyde family (Figure 23.3c.).
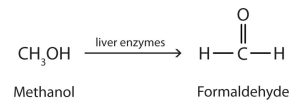
Formaldehyde reacts rapidly with the components of cells, coagulating proteins in much the same way that cooking coagulates an egg. This property of formaldehyde accounts for much of the toxicity of methanol.
Ethanol is oxidized in the liver to acetaldehyde (ethanal) (Figure 23.3d.).
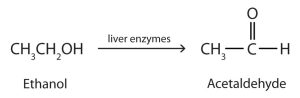
The acetaldehyde is in turn oxidized to acetic acid (HC2H3O2), a normal constituent of cells, which is then oxidized to carbon dioxide and water. Even so, ethanol is potentially toxic to humans. The rapid ingestion of 1 pt (about 500 mL) of pure ethanol would kill most people, and acute ethanol poisoning kills several hundred people each year. Ethanol freely crosses into the brain, where it depresses the respiratory control center, resulting in failure of the respiratory muscles in the lungs and hence suffocation. Ethanol is believed to act on nerve cell membranes, causing a diminution in speech, thought, cognition, and judgment.
Rubbing alcohol is usually a 70% aqueous solution of isopropyl alcohol (propan-2-ol). It has a high vapor pressure, and its rapid evaporation from the skin produces a cooling effect. It is toxic when ingested but compared to methanol, is less readily absorbed through the skin.
Hydration of Alkenes
Many simple alcohols are made by the hydration of alkenes. Ethanol is made by the hydration of ethylene in the presence of a catalyst such as sulfuric acid (H2SO4) (Figure 23.3e.).
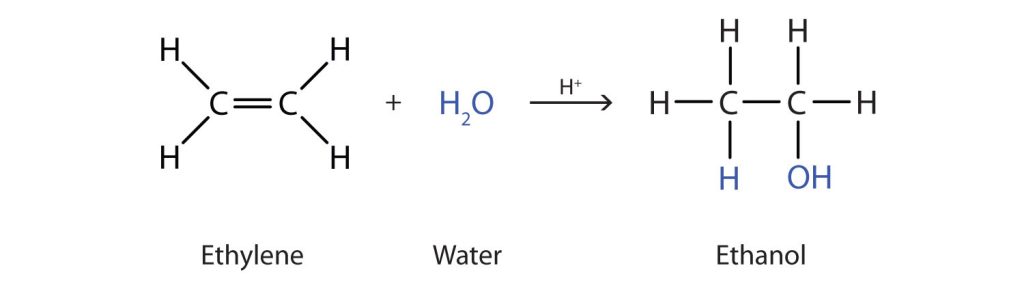
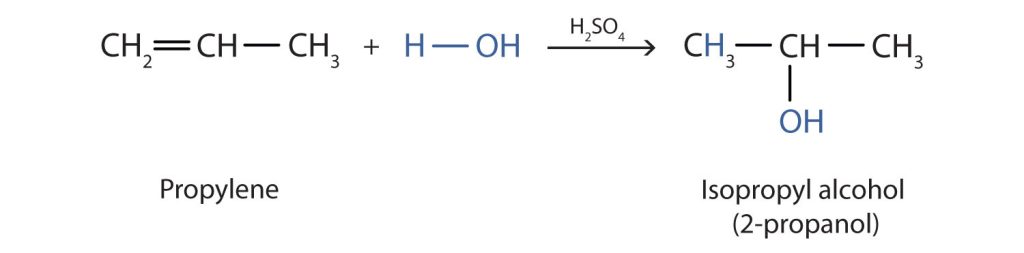
In a similar manner, isopropyl alcohol (2-propanol) is produced by the addition of water to propene (propylene) (Figure 23.3f.). In this reaction, Markovnikov's Rule applies to the carbon-carbon double bond addition (see details in Section 22.3). The -OH group of the water molecule will attach to the carbon of the carbon-carbon double bond that has more alkyl substituents. The H of the water molecule will attach to the carbon with the fewer alkyl substituents. Thus, the -OH group will attach to carbon 2 rather than carbon 2.
Write the equation for the reaction of 2-butene with water to form an alcohol. What alcohol is formed? Indicate that sulfuric acid is used as a catalyst.
Solution
First write the condensed structural formula of 2-butene and indicate that it reacts with water. When water adds to the carbon-carbon double bond, 2-butanol is formed. Then write the condensed structural formula of 2-butanol after the reaction arrow to indicate that it is the product. Finally, write the formula for the catalyst above the arrow.
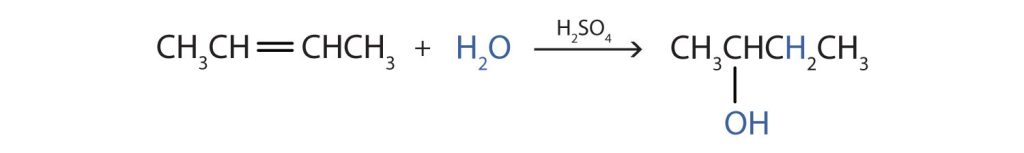
Example source: Adapted by Samantha Sullivan Sauer from Introduction to Chemistry: GOB(V. 1.0)., CC BY-NC-SA 3.0.
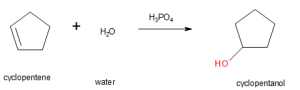
Write the equation for the reaction of cyclopentene with water to form an alcohol. What alcohol is formed? Indicate that phosphoric acid (H3PO4) is used as a catalyst.
Check Your Answer:[1]
Exercise source: Adapted by Samantha Sullivan Sauer from Introduction to Chemistry: GOB(V. 1.0)., CC BY-NC-SA 3.0, using images from Biovia Draw, licensed under CC BY-NC 4.0
Other Alcohol Producing Reactions
Alkyl Halide Substitution
In this substitution reaction, an alkyl halide is reacted with sodium hydroxide (NaOH) or potassium (KOH) for form an alcohol. This works for producing primary and, occasionally, secondary alcohols only (Figure 23.3g.). A by-product of sodium or potassium salt is formed. ("Alcohol (chemistry)", 2024).

Ester Hydrolysis
An ester is the product of an alcohol and a carboxylic acid. When this reaction is reversed, an alcohol is produced. This process will be explained in further detail in Chapter 25.
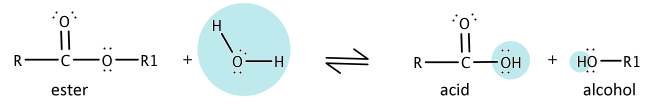
Reduction of Aldehydes and Ketones
An aldehyde or ketone, when reduced, will form a primary or secondary alcohol respectively (Figure 23.3i.). This process will be explained in further detail in Chapter 24.

Attribution & References
Except where otherwise noted, this page is written and adapted by David Wegman and Samantha Sullivan Sauer from:
- "14.4: Reactions that Form Alcohols" In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- "17.5: Alcohols from Carbonyl Compounds- Reduction" In Organic Chemistry (OpenStax via LibreTexts) by John McMurray, a LibreTexts version of Organic Chemistry (OpenStax). Access for free at Organic Chemistry (OpenStax).
- "18.2 Alcohols and Ethers" In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax).
References cited in-text
Alcohol (chemistry). (2024, January 26). In Wikipedia.
- cyclopentanol is formed in this reaction.
 ↵
↵

