25.2 Physical Properties of Carboxylic Acids
Learning Objectives
By the end of this section, you will be able to:
- Compare the boiling points of carboxylic acids with alcohols of similar molar mass.
- Compare the solubilities of carboxylic acids in water with the solubilities of comparable alkanes and alcohols in water.
Many carboxylic acids are colourless liquids with disagreeable odours. The carboxylic acids with 5 to 10 carbon atoms all have “goaty” odours (explaining the odour of Limburger cheese). These acids are also produced by the action of skin bacteria on human sebum (skin oils), which accounts for the odour of poorly ventilated locker rooms. The acids with more than 10 carbon atoms are waxlike solids, and their odour diminishes with increasing molar mass and resultant decreasing volatility.
Carboxylic acids exhibit strong hydrogen bonding between molecules. They therefore have high boiling points compared to other substances of comparable molar mass.
The carboxyl group readily engages in hydrogen bonding with water molecules (Figure 25.2a.). The acids with one to four carbon atoms are completely miscible with water. Solubility decreases as the carbon chain length increases because dipole forces become less important and dispersion forces become more predominant. Hexanoic acid [CH3(CH2)4COOH] is barely soluble in water (about 1.0 g/100 g of water). Palmitic acid [CH3(CH2)14COOH], with its large nonpolar hydrocarbon component, is essentially insoluble in water. The carboxylic acids generally are soluble in such organic solvents as ethanol, toluene, and diethyl ether.
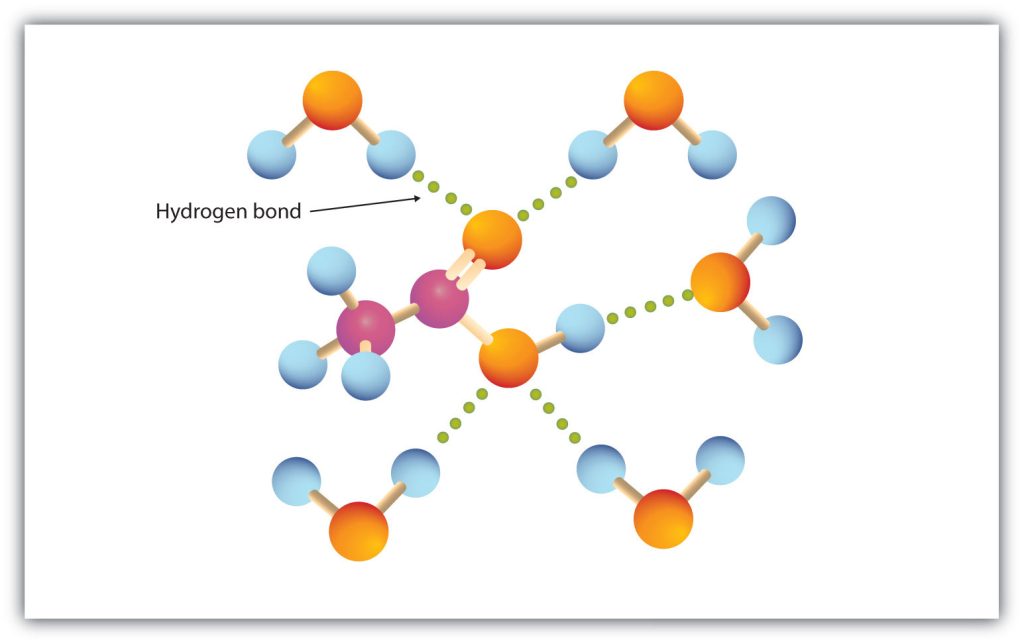
Table 25.2a. gives a summary of the physical properties for selected carboxylic acids.
| Condensed Structural Formula | Name of Acid | Melting Point (°C) | Boiling Point (°C) | Solubility (g/100 g of Water) |
|---|---|---|---|---|
| HCOOH | formic acid | 8 | 100 | miscible |
| CH3COOH | acetic acid | 17 | 118 | miscible |
| CH3CH2COOH | propionic acid | –22 | 141 | miscible |
| CH3(CH2)2COOH | butyric acid | –5 | 163 | miscible |
| CH3(CH2)3COOH | valeric acid | –35 | 187 | 5 |
| CH3(CH2)4COOH | caproic acid | –3 | 205 | 1.1 |
| C6H5COOH | benzoic acid | 122 | 249 | 0.29 |
Table source: “15.3: Physical Properties of Carboxylic Acids” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
Spotlight on Everyday Chemistry: Maksim Fomich’s Research on Deuterated Fatty Acids
Maksim Fomich is currently looking into creating deuterated polyunsaturated fatty acid compounds, with a view to using them to potentially treat a range of diseases. Here, he explains the premise behind his research.

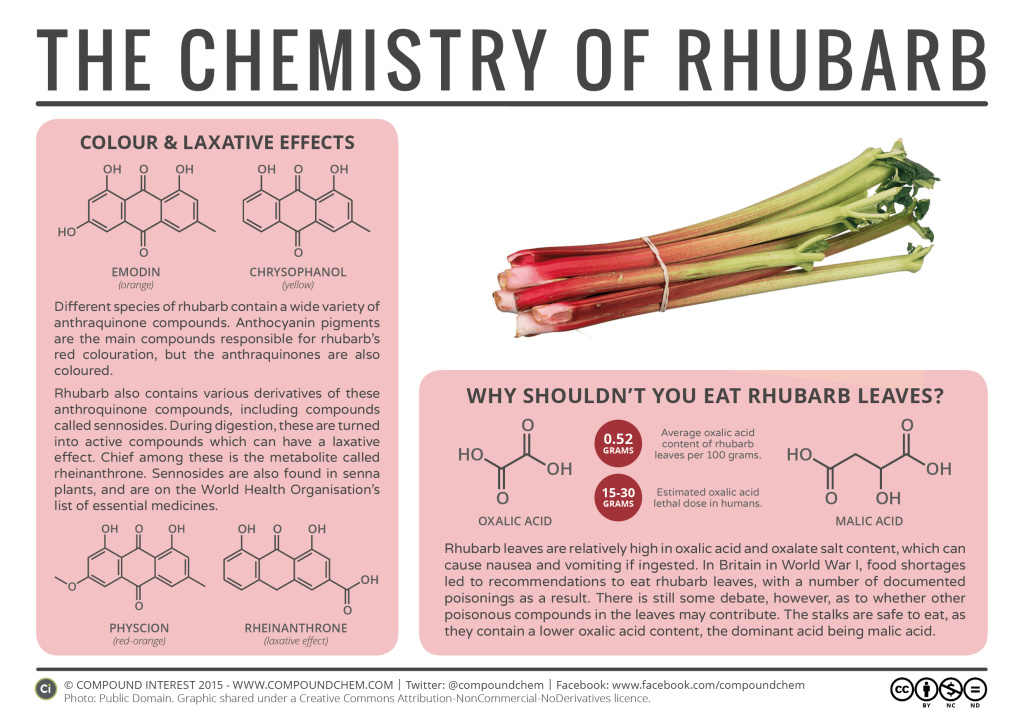
Spotlight on Everyday Chemistry: The Chemistry of Rhubarb
Oxalic acid, an organic compound found in rhubarb leaves, is an example of a dicarboxylic acid. In fact it is the simplest dicarboxylic acid found and is a white crystalline solid that forms a colourless solution in water. Oxalic acid is one component of rhubarb leaves that can be hard on the human stomach if ingested.
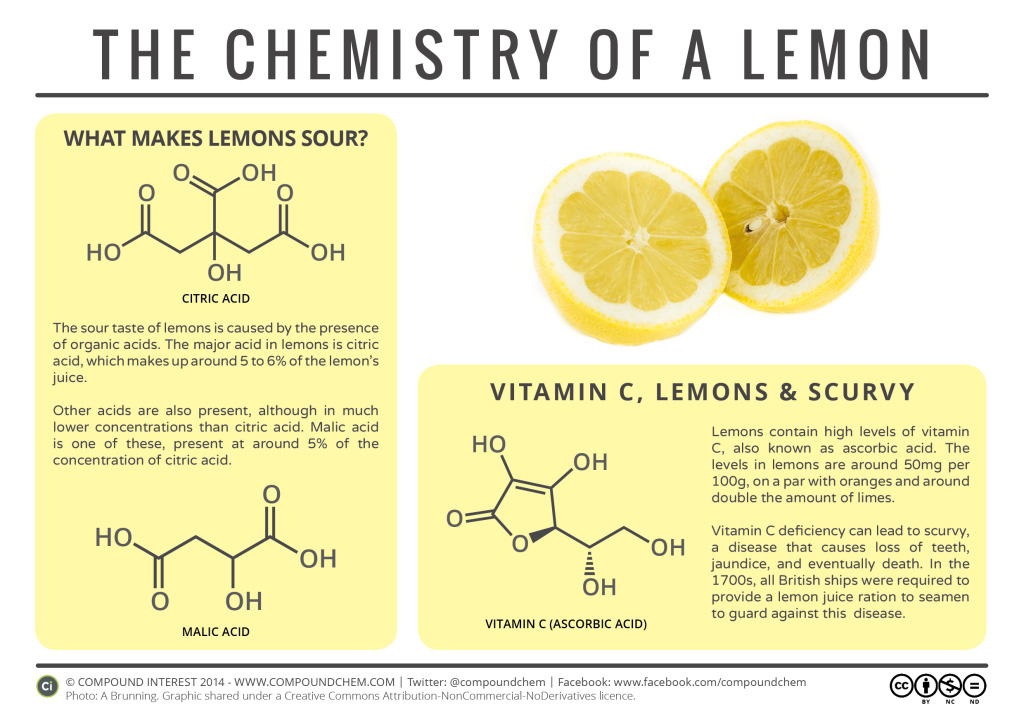
Spotlight on Everyday Chemistry: The Chemistry of a Lemon
The sour taste of lemons is due in fact to the presence of carboxylic acids. Citric acid is considered to be a tricarboxylic acid, that has a role as a food acidity regulator, an antimicrobial agent and a fundamental metabolite. Malic acid is a dicarboxylic acid that contributes to the sour taste of fruits, plays a role as a food acidity regulator and can be used as a food additive.
Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey from “15.3: Physical Properties of Carboxylic Acids” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0. / A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.

