24.3 Physical Properties of Aldehydes and Ketones
Learning Objectives
By the end of this section, you will be able to:
- Explain why the boiling points of aldehydes and ketones are higher than those of ethers and alkanes of similar molar masses but lower than those of comparable alcohols.
- Compare the solubilities in water of aldehydes and ketones of four or fewer carbon atoms with the solubilities of comparable alkanes and alcohols.
- Identify common aldehydes and ketones
- Understand the implications of these common aldehydes and ketones in our daily lives
Bonding of Aldehydes and Ketones
The carbon-to-oxygen double bond is quite polar, more polar than a carbon-to-oxygen single bond. The electronegative oxygen atom has a much greater attraction for the bonding electron pairs than does the carbon atom. The carbon atom has a partial positive charge, and the oxygen atom has a partial negative charge, as shown in Figure 24.3a.
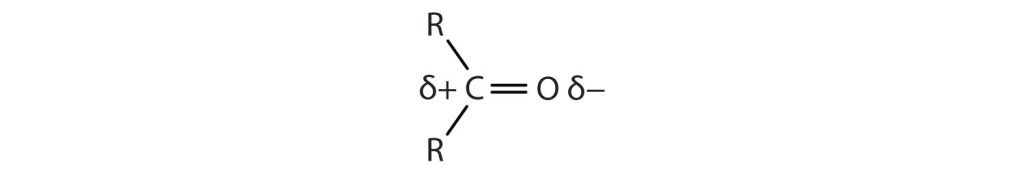
Physical Properties of Aldehydes and Ketones
In aldehydes and ketones, this charge separation leads to dipole-dipole interactions that are great enough to significantly affect the boiling points. Table 24.3a. shows that the slightly polar single bonds in ethers have little such effect, whereas hydrogen bonding between alcohol molecules is even stronger.
| Compound | Family | Molar Mass | Type of Intermolecular Forces | Boiling Point (°C) |
|---|---|---|---|---|
| CH3CH2CH2CH3 | alkane | 58 | dispersion only | –1 |
| CH3OCH2CH3 | ether | 60 | weak dipole | 6 |
| CH3CH2CHO | aldehyde | 58 | strong dipole | 49 |
| CH3CH2CH2OH | alcohol | 60 | hydrogen bonding | 97 |
Table source: “14.10: Properties of Aldehydes and Ketones” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
Methanal (common name: formaldehyde) is a gas at room temperature. Ethanal (common name: acetaldehyde) boils at 20°C; in an open vessel, it boils away in a warm room. Most other common aldehydes are liquids at room temperature.
Although the lower members of the homologous series have pungent odours, many higher molar mass aldehydes have pleasant odours and are used in perfumes and artificial flavourings. As for the ketones, propanone (common name: acetone) has a pleasant odour, but most of the higher molar mass ketones have rather bland odours.
The oxygen atom of the carbonyl group engages in hydrogen bonding with a water molecule, as shown in Figure 24.3b.
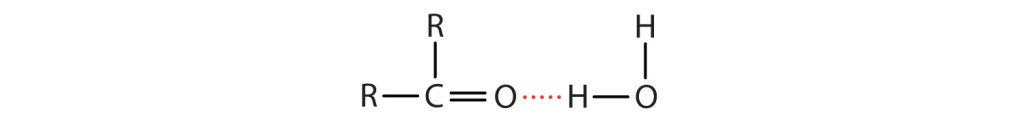
The solubility of aldehydes is therefore about the same as that of alcohols and ethers. Methanal, ethanal, and propanone are soluble in water. As the carbon chain increases in length, solubility in water decreases, as dispersion forces become stronger. The borderline of solubility occurs at about four carbon atoms per oxygen atom. All aldehydes and ketones are soluble in organic solvents and, in general, are less dense than water.
Spotlight on Everyday Chemistry: The Chemistry of Cloves and Coriander
Aldehyde and ketone functional groups are very common in everyday materials. In these two examples, cloves (Infographic 24.3a.) and coriander (Infographic 24.3b.) are highlighted for the importance of the carbonyl functional group in the aromas.
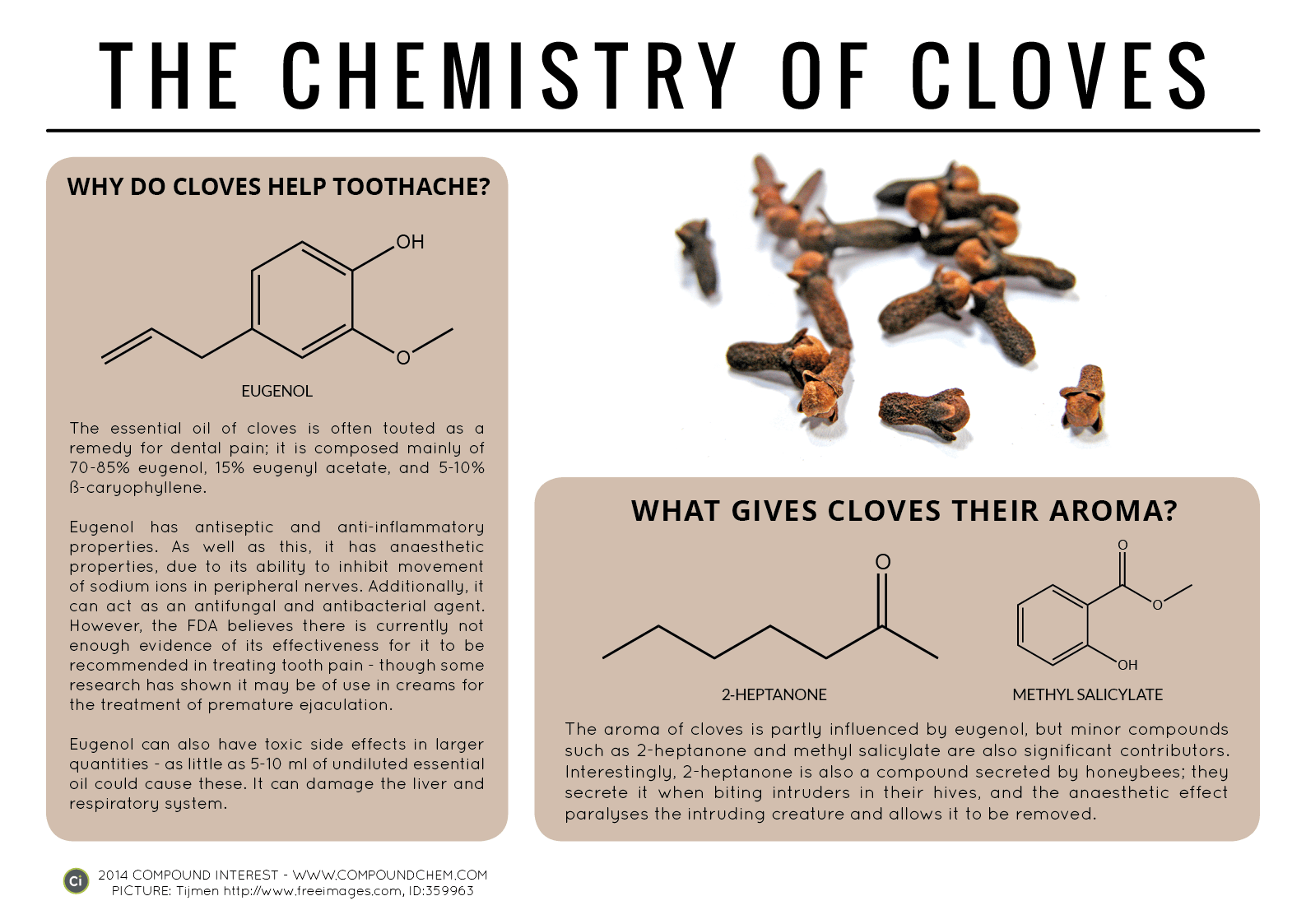
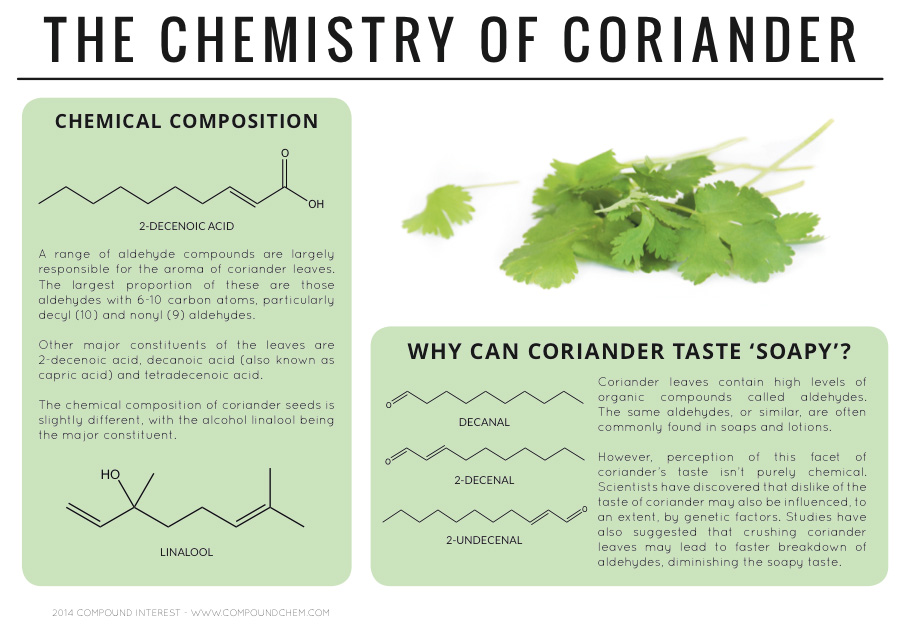
Common Aldehydes and Ketones
Formaldehyde (methanal) has an irritating odour. Because of its reactivity, it is difficult to handle in the gaseous state. For many uses, it is therefore dissolved in water and sold as a 37% to 40% aqueous solution called formalin. Formaldehyde denatures proteins, rendering them insoluble in water and resistant to bacterial decay. For this reason, formalin is used in embalming solutions and in preserving biological specimens.
Aldehydes are the active components in many other familiar substances. Large quantities of formaldehyde are used to make phenol-formaldehyde resins for gluing the wood sheets in plywood and as adhesives in other building materials. Sometimes the formaldehyde escapes from the materials and causes health problems in some people. While some people seem unaffected, others experience coughing, wheezing, eye irritation, and other symptoms. The odour of green leaves is due in part to a carbonyl compound, cis-3-hexenal, which with related compounds is used to impart a “green” herbal odour to shampoos and other products.
Acetaldehyde (ethanal) is an extremely volatile, colourless liquid. It is a starting material for the preparation of many other organic compounds, namely acetic acid and 1-butanol, both of which are extremely valuable industrial products. Biologically speaking, acetaldehyde is formed as a metabolite in the fermentation of sugars and in the detoxification of alcohol in the liver. For this latter reason, it is worth noting that it is acetaldehyde that causes the negative physiological impacts associated with heavy alcohol consumption, as it is a toxin. It is produced in the liver during the first step of the detoxification process, and the reactivity of this functional group allows it to bond to many biochemicals. For example, acetaldehyde can react with amino acids to slow protein synthesis; react with antioxidants to increase damage to the liver; react with proteins to hamper the liver’s ability to export needed chemicals into the bloodstream. All of these side effects of alcohol consumption, and thus the production of acetaldehyde, can lead to liver cirrhosis in humans.
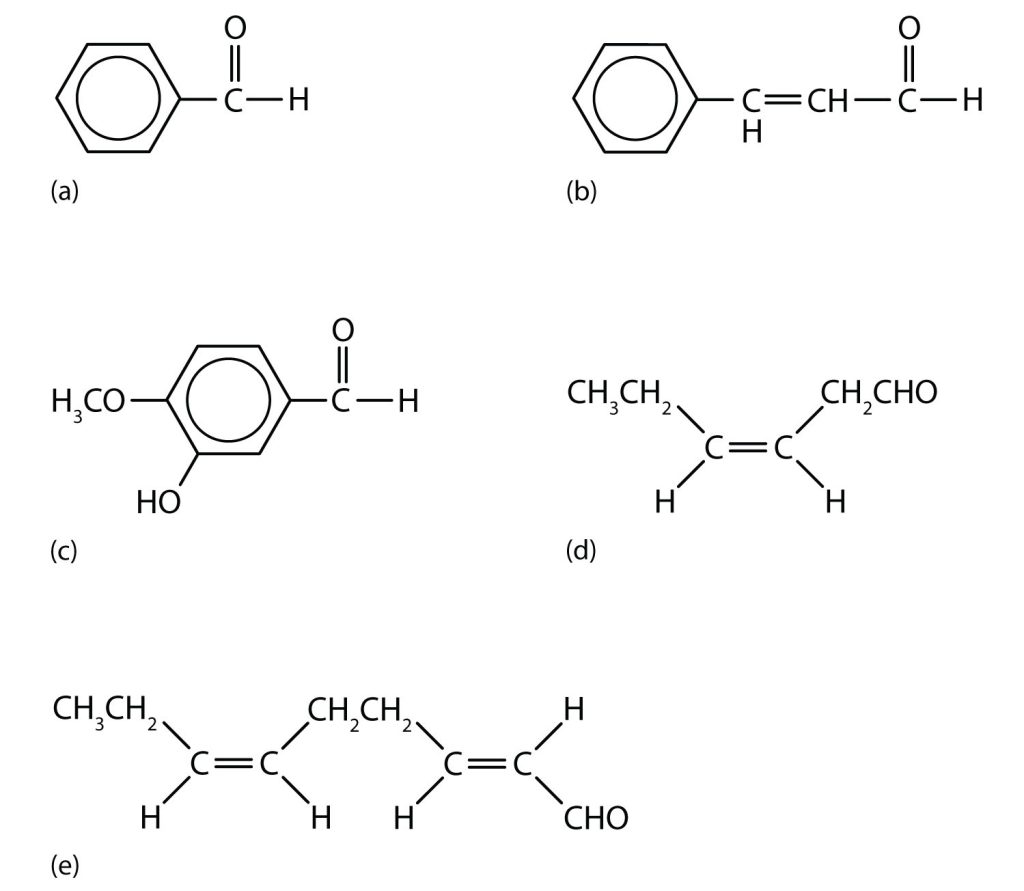
Aldehydes are the active components of many other familiar materials that we find in many food products, as shown in Figure 24.3c.
Acetone (propanone) is the simplest and most important ketone. Because it is miscible with water as well as with most organic solvents, its chief use is as an industrial solvent. Acetone is the main solvent used in the manufacture of drugs, explosives, various chemicals, and for the manufacture of plastics. It is also the chief ingredient in some brands of nail polish remover. Acetone is formed in the human body as a by-product of lipid metabolism. Normally, acetone does not accumulate to an appreciable extent because it is oxidized to carbon dioxide and water. The normal concentration of acetone in the human body is less than 1 mg/100 mL of blood. In certain disease states, such as uncontrolled diabetes mellitus, the acetone concentration rises to higher levels. It is then excreted in the urine, where it is easily detected. In severe cases, its odour can be noted on the breath (this is how doctors once diagnosed the disease).
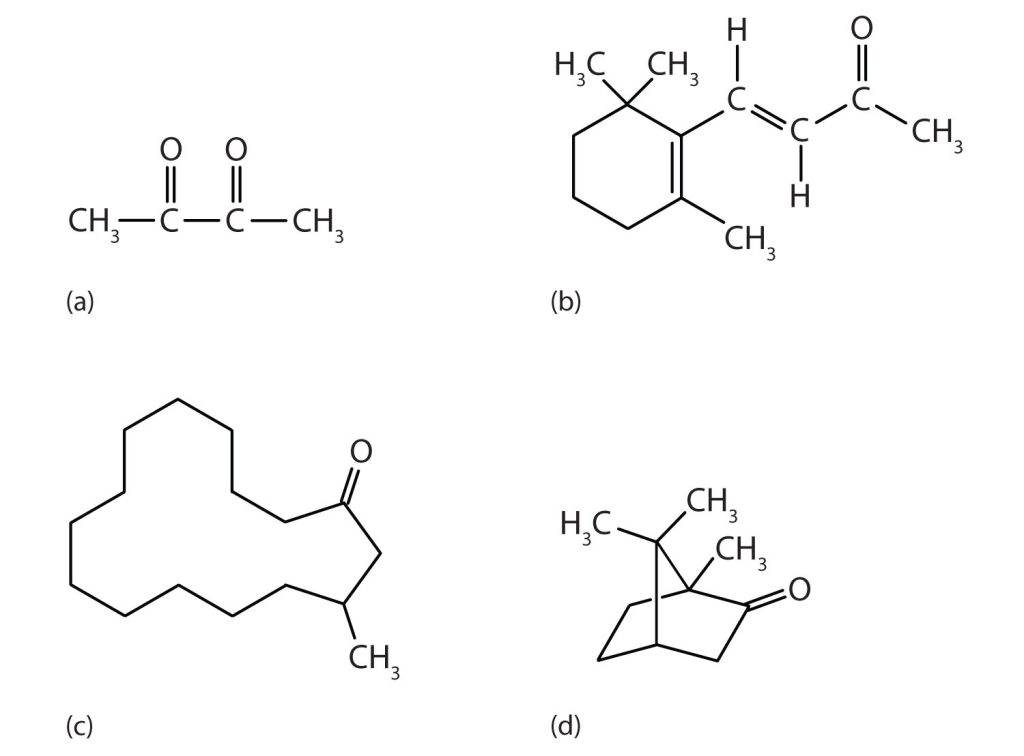
Methyl ethyl ketone (2-butanone, or MEK) is a major solvent used to produce paints and lacquers. Ketones are also the active components of other familiar substances, some of which are shown in Figure 24.3d.
Aldehydes and ketones are widespread in nature and are often combined with other functional groups. Examples of naturally occurring molecules which contain an aldehyde or ketone functional group are shown in Figure 24.3e. and 24.3f. Many of these molecular structures are chiral.
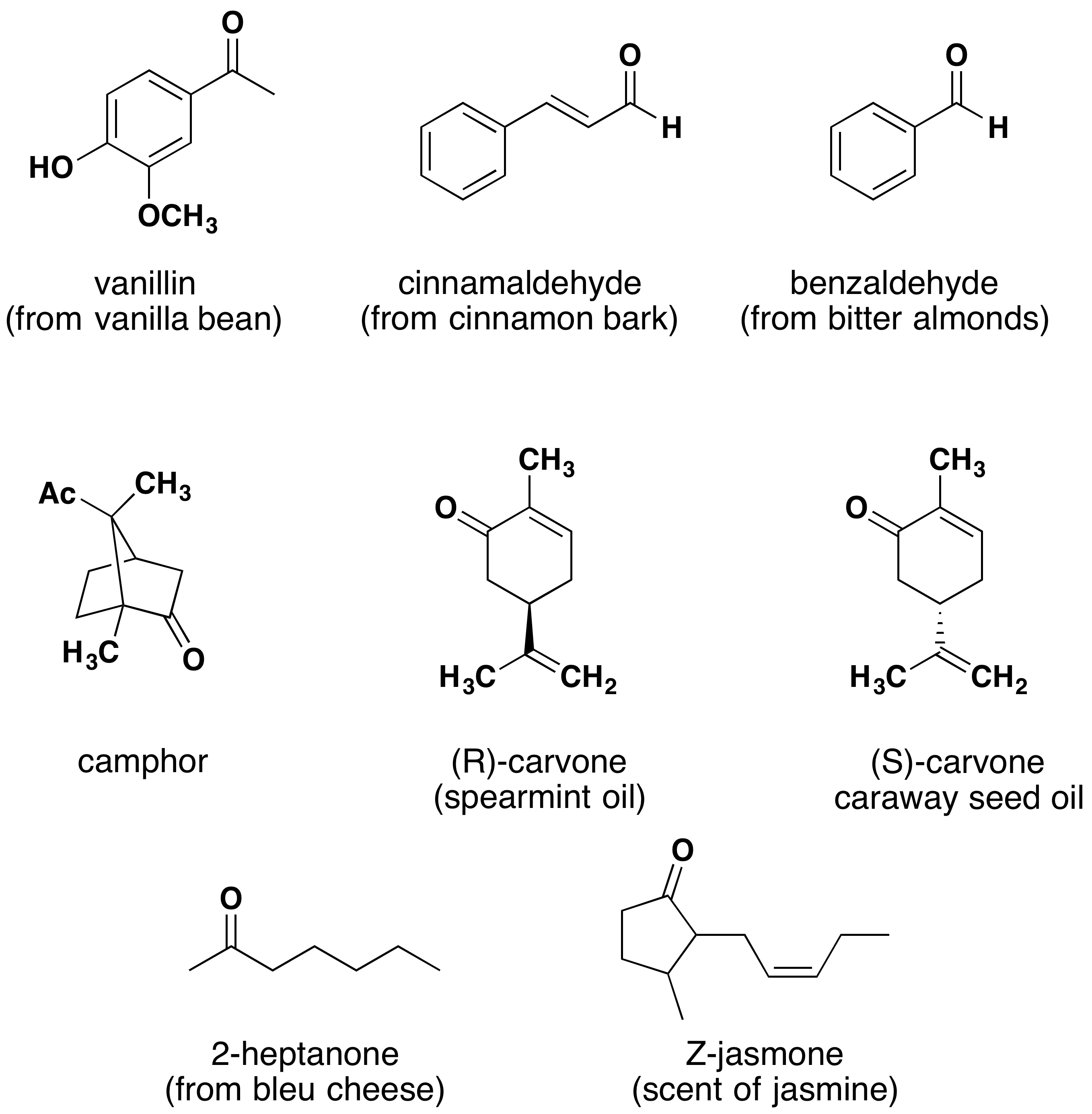
When chiral compounds are found in nature, they are usually enantiomerically pure, although different sources may yield different enantiomers. For example, carvone is found as its levorotatory (R)-enantiomer in spearmint oil, whereas caraway seeds contain the dextrorotatory (S)-enantiomer. In this case the change of the stereochemistry causes a drastic change in the perceived scent. Aldehydes and ketones are known for their sweet and sometimes pungent odours. The odour from vanilla extract comes from the molecule vanillin. Likewise, benzaldehyde provides a strong scent of almonds. Because of their pleasant fragrances aldehyde and ketone containing molecules are often found in perfumes. However, not all of the fragrances are pleasing. In particular, 2-Heptanone provides part of the sharp scent from blue cheese and (R)-Muscone is part of the musky smell from the Himalayan musk deer.
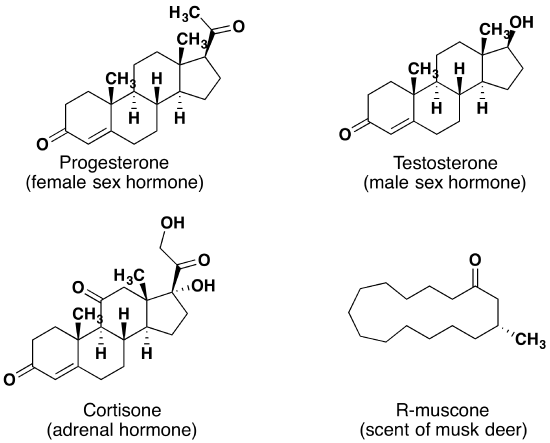
Lastly, ketones show up in many important hormones such as progesterone (a female sex hormone) and testosterone (a male sex hormone). Progesterone is a hormone secreted by the ovaries that stimulates the growth of cells in the uterine wall and prepares it for attachment of a fertilized egg, and testosterone is the main male sex hormone. These and other sex hormones affect our development and our lives in fundamental ways. Notice how subtle differences in structure can cause drastic changes in biological activity. The ketone functionality also shows up in the anti-inflammatory steroid, Cortisone.
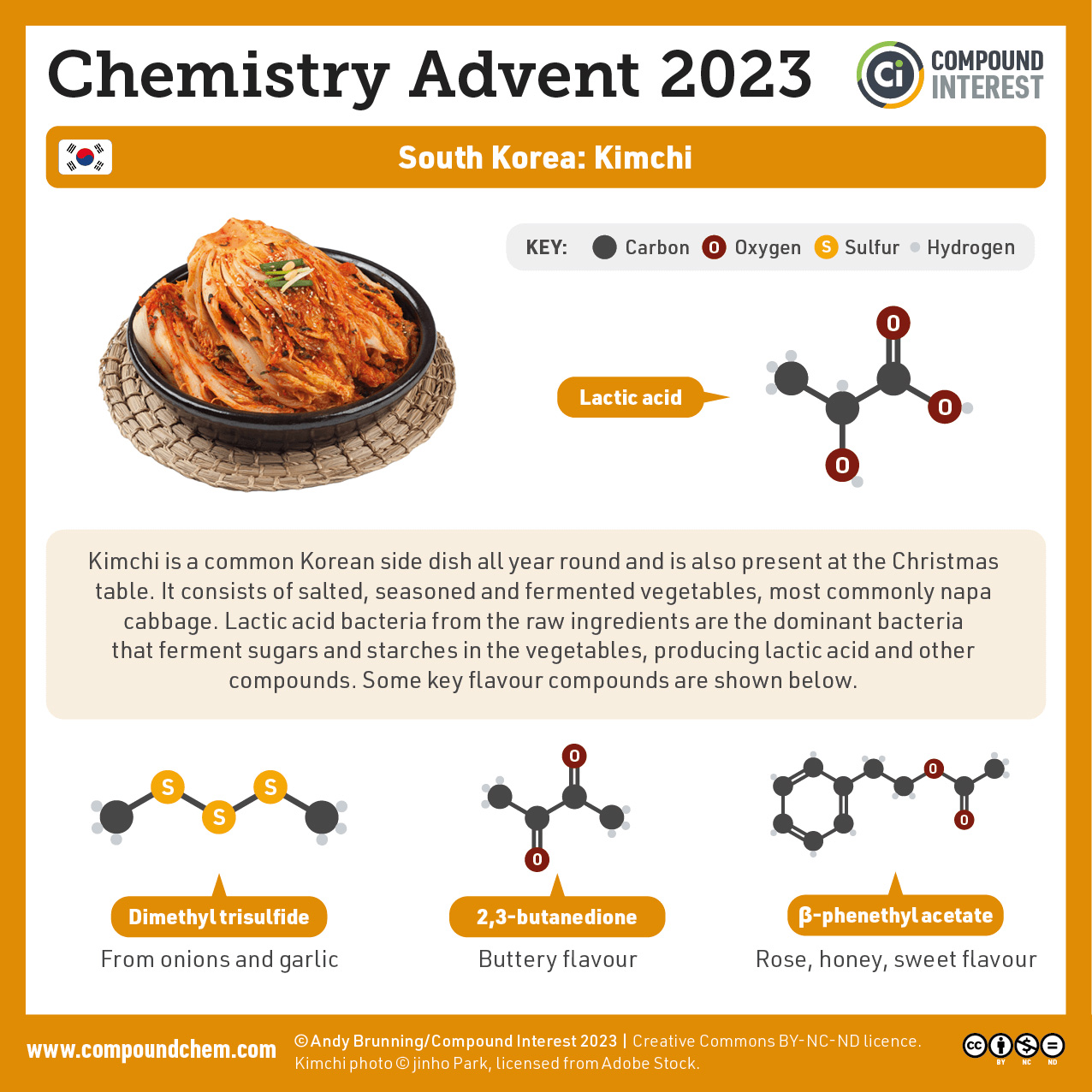
Indigenous Perspectives: Kimchi (South Korea)
Kimchi is a traditional dish of the Korean people that dates back to the Goryeo Dynasty (about 1000 AD) (Kaner-White, 2023). One of the flavours in this vegetable dish results from a dione, 2,3-butanedione giving a buttery flavour. See Infographic 24.3c. for more details.
Attribution & References
Except where otherwise noted, this page is adapted by Gregory A. Anderson and Samantha Sullivan Sauer from
- “15.3: Properties of Aldehydes and Ketones” & “15.4: Some Common Aldehydes and Ketones” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0
- “Natural Occurrence of Aldehydes and Ketones” by Steven Farmer & William Reusch In Supplemental Modules (Organic Chemistry), CC BY-NC-SA 4.0
- “14.10: Properties of Aldehydes and Ketones” In Basics of General, Organic, and Biological Chemistry (Ball et al.)by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
References cited in-text
Kaner-White, Y. (2023, June 3). What is kimchi, exactly? A tangy history of the Korean dish. Readers Digest.

