24.2 Naming Aldehydes and Ketones
Learning Objectives
By the end of this section, you will be able to:
- Use the IUPAC system to name and draw aldehydes and ketones
- Use common names to name low molecular weight aldehydes and ketones
Naming aldehydes and ketones using IUPAC rules
When following the preferred International Union of Pure and Applied Chemistry (IUPAC) rules for naming either an aldehyde or a ketone, several steps must be followed. The following are the IUPAC rules for naming aldehydes and ketones:
- The stem names of aldehydes and ketones are derived from those of the parent alkanes, defined by the longest continuous chain (LCC) of carbon atoms that contains the functional group.
- For an aldehyde, drop the –e from the alkane name and add the ending –al. Methanal is the IUPAC name for formaldehyde, and ethanal is the name for acetaldehyde.
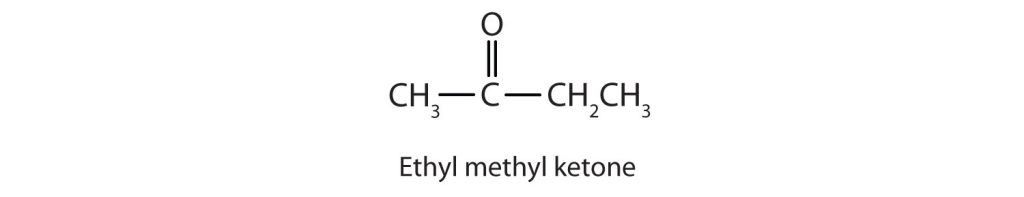
- For a ketone, drop the –e from the alkane name and add the ending –one. Propanone is the IUPAC name for acetone, and butanone is the name for ethyl methyl ketone.
- To indicate the position of a substituent on an aldehyde, the carbonyl carbon atom is always considered to be C1; it is unnecessary to designate this group by number.
- To indicate the position of a substituent on a ketone, number the chain in the manner that gives the carbonyl carbon atom the lowest possible number. In cyclic ketones, it is understood that the carbonyl carbon atom is C1.
Exercise 24.2a
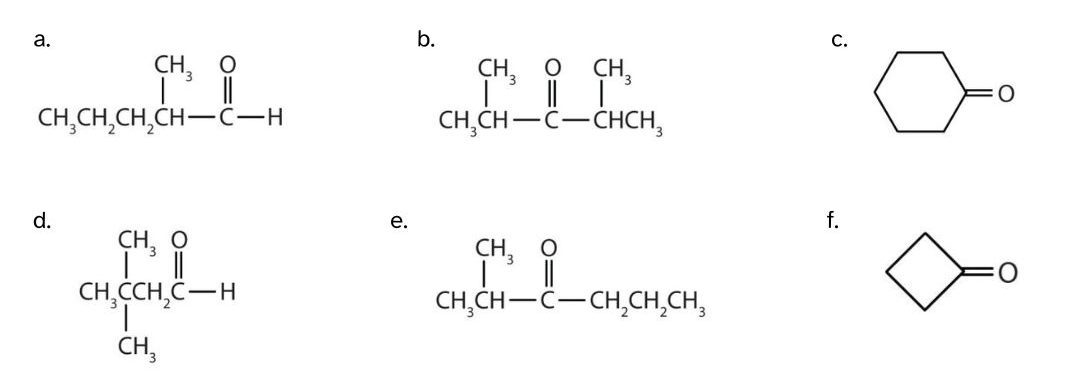
Give the IUPAC name for each compound.
Check Your Answer[1]
Source: Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0.
Naming aldehydes and ketones using common names
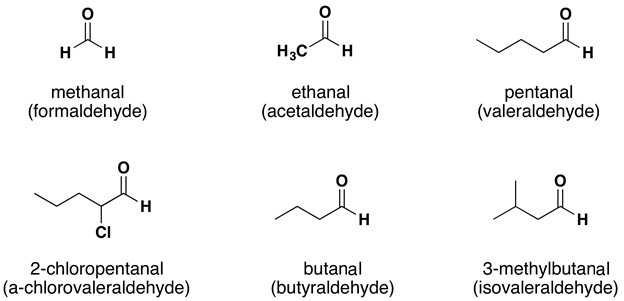
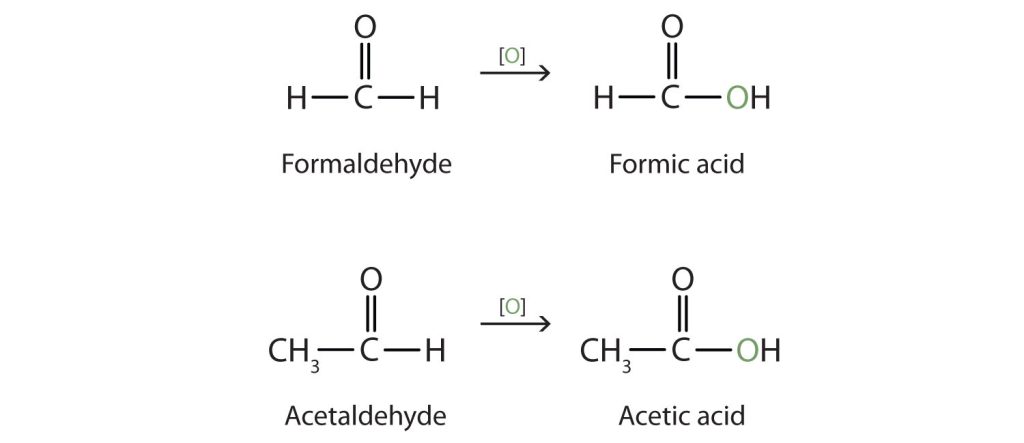
Both common and International Union of Pure and Applied Chemistry (IUPAC) names are frequently used for aldehydes and ketones, with common names predominating for the lower molecular weight molecules. The common names of aldehydes are taken from the names of the acids into which the aldehydes can be converted by oxidation (Figure 24.2c.).
The stems for the common names of the first four aldehydes are as follows:
- 1 carbon atom: form–
- 2 carbon atoms: acet–
- 3 carbon atoms: propion–
- 4 carbon atoms: butyr–
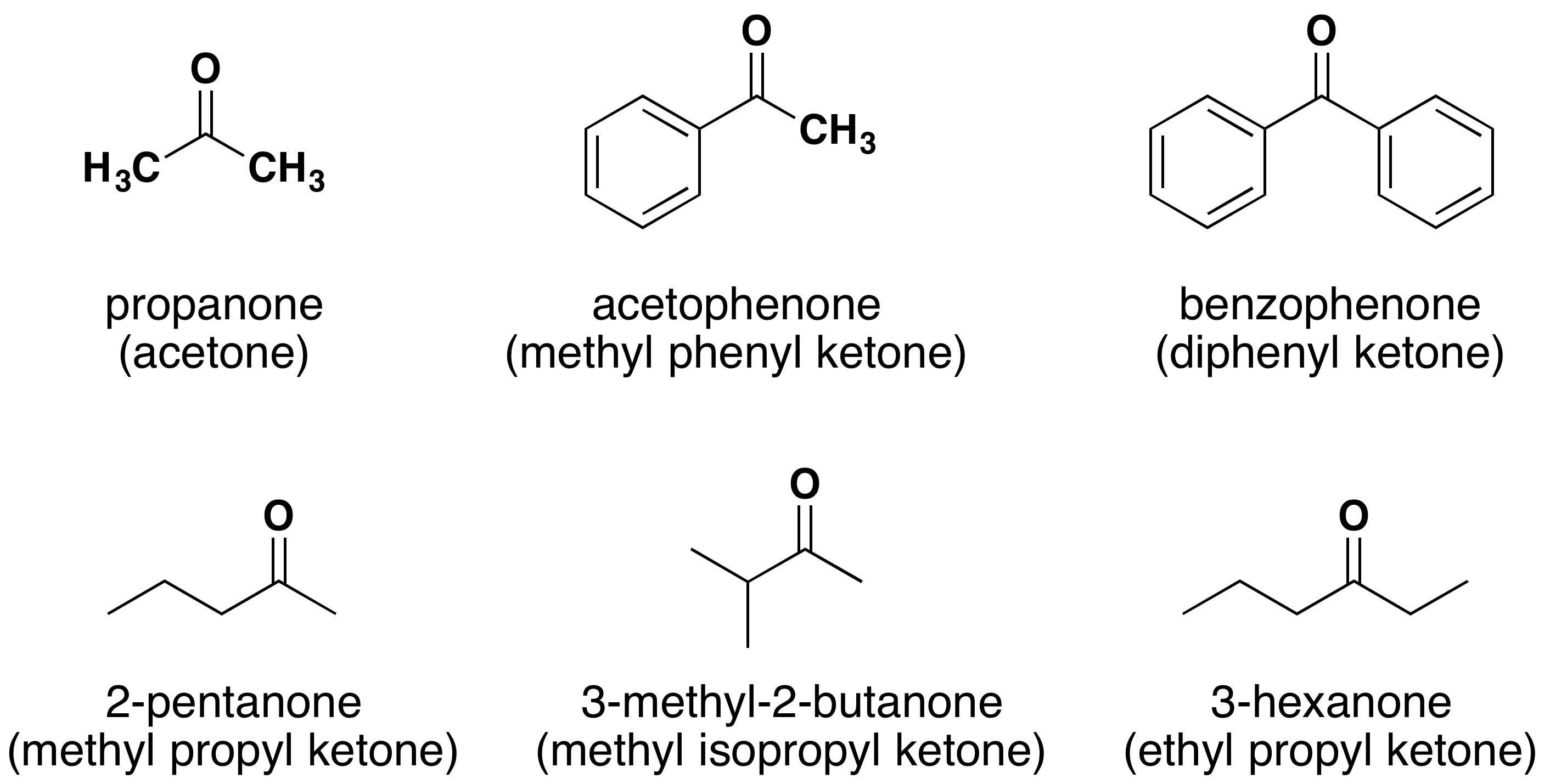
Because the carbonyl group in a ketone must be attached to two carbon groups, the simplest ketone has three carbon atoms. It is widely known as acetone, a unique name unrelated to other common names for ketones (Figure 24.2d.).
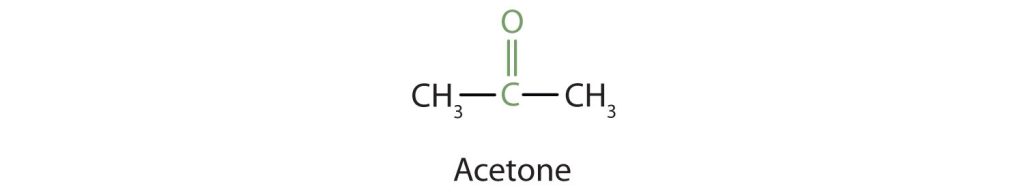
Generally, the common names of ketones consist of the names of the groups attached to the carbonyl group, followed by the word ketone. (Note the similarity to the naming of ethers.) Another name for acetone, then, is dimethyl ketone. The ketone with four carbon atoms is ethyl methyl ketone (Figure 24.2e.).
Classify each compound as an aldehyde or a ketone and give the common name for each.
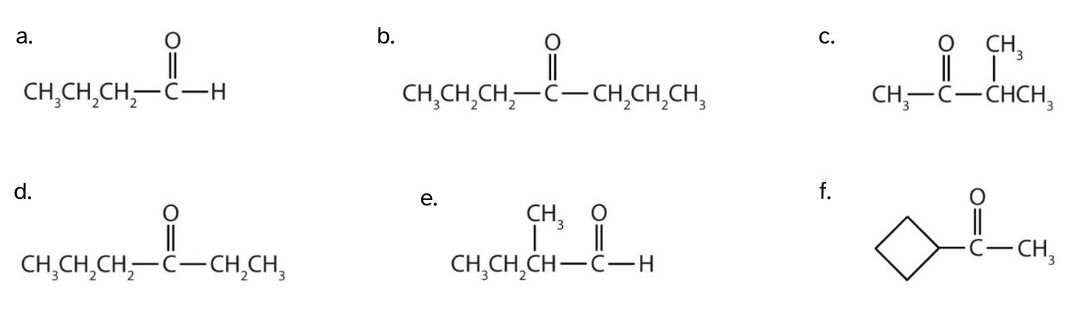
Check Your Answer[2]
Drawing structures for aldehydes and ketones
When it comes to drawing molecular structures for aldehydes and ketones, the best practice is to follow similar rules to what we’ve seen in previous chapters: work your way from right-to-left in the name. Start by drawing a skeleton structure for the parent compound, number your compound, and then add side groups as a final step.
Draw the structure for each compound.
- 7-chlorooctanal
- 4-methyl–3-hexanone
Check Your Answer:[3]
Carbonyl plus Other Functional Groups in Same Molecule
As with many molecules with two or more functional groups, one is given priority while the other is named as a substituent. When an aldehyde or ketone is present in a molecule which also contains an alcohol functional group, the carbonyl is given nomenclature priority by the IUPAC system. This means that the carbonyl is given the lowest possible location number and the appropriate nomenclature suffix is included. In the case of the alcohols, the OH is named as a hydroxyl substituent (Figure 24.2f.).
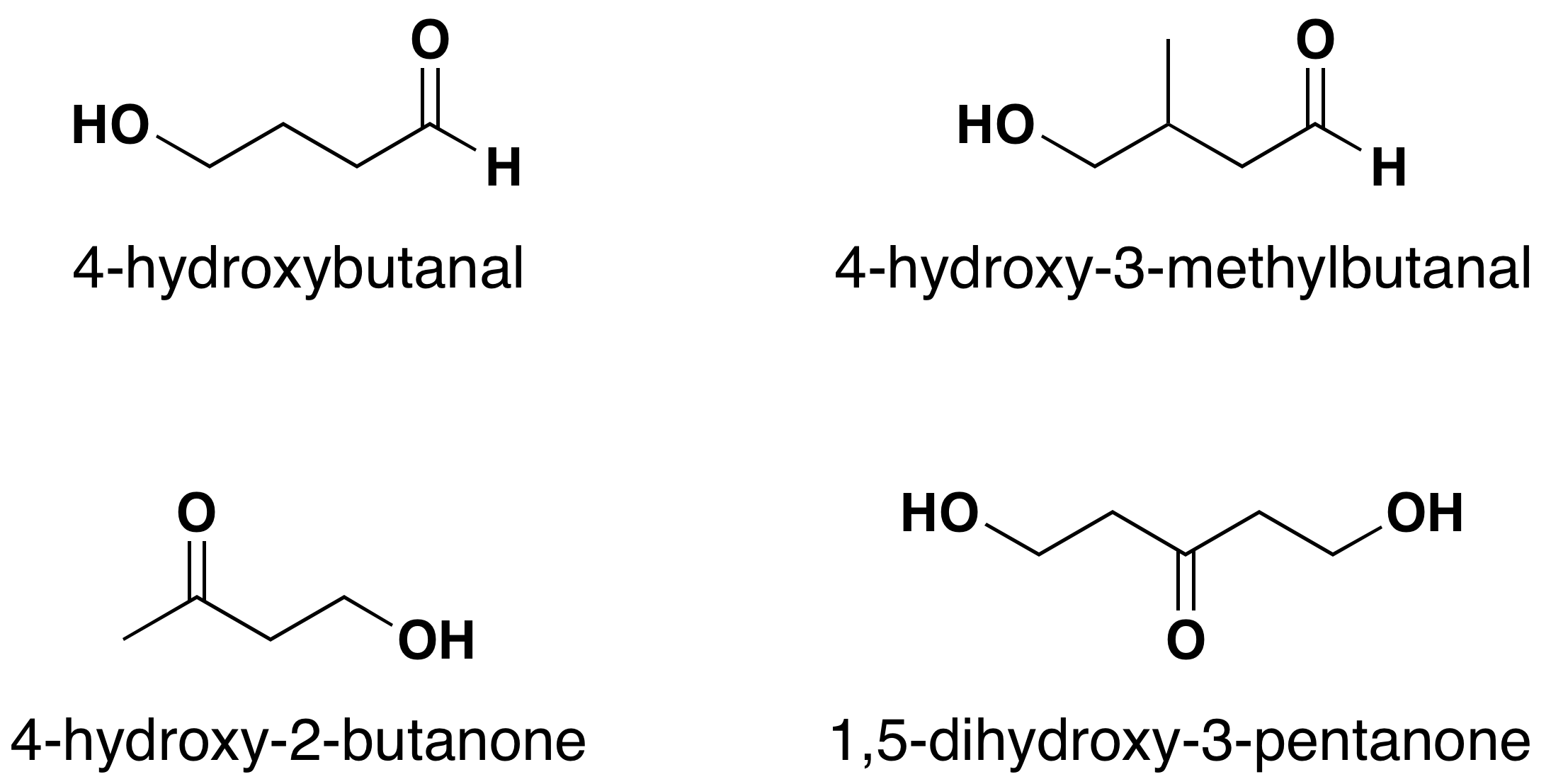
When and aldehyde or ketone is present in a molecule which also contains an alkene functional group the carbonyl is given nomenclature priority by the IUPAC system. This means that the carbonyl is given the lowest possible location number, and the appropriate nomenclature suffix is included. When carbonyls are included with an alkene the following order is followed:
(Location number of the alkene)-(Prefix name for the longest carbon chain minus the -ane ending)-(an -en ending to indicate the presence of an alkene)-(the location number of the carbonyl if a ketone is present)-(either an –one or and -anal ending).
Remember that the carbonyl has priority so it should get the lowest possible location number. Also, remember that cis/tran or E/Z nomenclature for the alkene needs to be included if necessary.
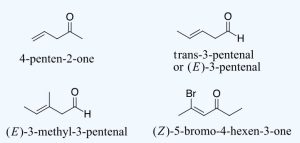
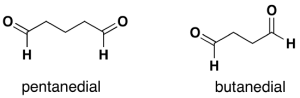
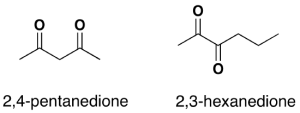
For dialdehydes, the location numbers for both carbonyls are omitted because the aldehyde functional groups are expected to occupy the ends of the parent chain (Figure 24.2h.). The ending –dial is added to the end of the parent chain name. For diketones, both carbonyls require a location number (Figure 24.2i.). The ending -dione or -dial is added to the end of the parent chain.
Links to Enhanced Learning
For more practice with naming and drawing ketones and aldehydes, see Nomenclature of Aldehydes & Ketones – Chemistry LibreTexts.
Attribution & References
Except where otherwise noted, this page is adapted by Gregory A. Anderson and Samantha Sullivan Sauer from
- “15.2: Naming Aldehydes and Ketones” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0
- “14.9: Aldehydes and Ketones- Structure and Names” In Basics of General, Organic, and Biological Chemistry (Ball et al.)by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- Carbonyl plus Other Functional Groups in Same Molecule section from “Nomenclature of Aldehydes & Ketones” by Steven Farmer & William Reusch In Supplemental Modules (Organic Chemistry), CC BY-NC-SA 4.0.
References from original source:- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry. 5th ed. New York: W.H. Freeman, 2007.
- Zumdahl, Steven S., and Susan A. Zumdahl. Chemistry. 6th ed. Boston: Houghton Mifflin College Division, 2002.
-
- There are five carbon atoms in the LCC. The methyl group (CH3) is a substituent on the second carbon atom of the chain; the aldehyde carbon atom is always C1. The name is derived from pentane. Dropping the -e and adding the ending -al gives pentanal. The methyl group on the second carbon atom makes the name 2-methylpentanal.
- There are five carbon atoms in the LCC. The carbonyl carbon atom is C3, and there are methyl groups on C2 and C4. The IUPAC name is 2,4-dimethyl-3-pentanone.
- There are six carbon atoms in the ring. The IUPAC name is cyclohexanone. No number is needed to indicate the position of the carbonyl group because all six carbon atoms are equivalent.
- There are four carbon atoms in the LCC. There are two methyl groups on the third carbon atom of the chain; the aldehyde carbon atom is always C1. The IUPAC name is 3,3-dimethylbutanal.
- There are six carbon atoms on the LCC. The carbonyl carbon atom is C3, and there is a methyl group on C2. The IUPAC name is 2-methylhexanone.
- There are four carbon atoms in the ring. The IUPAC name is cyclobutanone.
↵
-
- This compound has the carbonyl group on an end carbon atom, so it is an aldehyde. The molecule is 4 carbons long, so its common name is butyraldehyde.
- This compound has the carbonyl group on an interior carbon atom, so it is a ketone. Both alkyl groups are propyl groups. The name is therefore dipropyl ketone.
- This compound has the carbonyl group between two alkyl groups, so it is a ketone. One alkyl group has three carbon atoms and is attached by the middle carbon atom; it is an isopropyl group. A group with one carbon atom is a methyl group. The name is therefore isopropyl methyl ketone.
- This compound has the carbonyl group on an interior carbon atom, so it is a ketone. One alkyl group has three carbon atoms and is attached by a terminal carbon atom; it is a propyl group. A group with two carbon atoms is an ethyl group. The name is therefore propyl ethyl ketone.
- This compound has the carbonyl group on an end carbon atom, so it is an aldehyde. The molecule is 4 carbons long, but the carbonyl group is attached to the secondary carbon in the chain; it is a sec-butyl group. The name is therefore sec-butylaldehyde.
- This compound has the carbonyl group on an interior carbon atom, so it is a ketone. One alkyl group is 4 carbons long in a ring structure; it is a cyclobutyl group. A group with one carbon atom is a methyl group. The common name is therefore cyclobutyl methyl ketone.
↵
-
- The octan- part of the name tells us that the LCC has eight carbon atoms. There is a chlorine (Cl) atom on the seventh carbon atom; numbering from the carbonyl group and counting the carbonyl carbon atom as C1, we place the Cl atom on the seventh carbon atom:
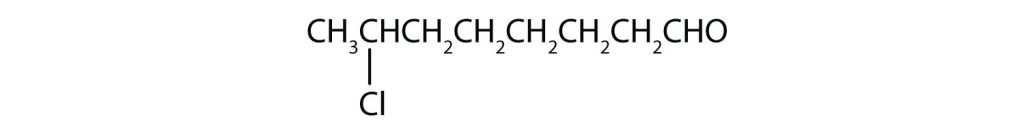
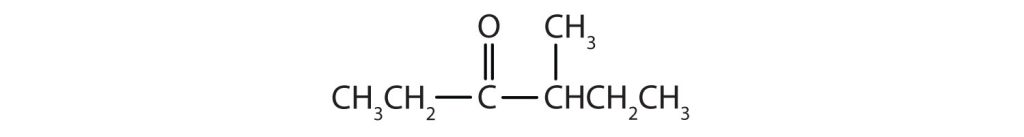
- The hexan- part of the name tells us that the LCC has six carbon atoms. The 3 means that the carbonyl carbon atom is C3 in this chain, and the 4 tells us that there is a methyl (CH3) group at C4:
- The octan- part of the name tells us that the LCC has eight carbon atoms. There is a chlorine (Cl) atom on the seventh carbon atom; numbering from the carbonyl group and counting the carbonyl carbon atom as C1, we place the Cl atom on the seventh carbon atom:

