25.3 Formation and Reactions of Carboxylic Acids
Learning Objectives
By the end of this section, you will be able to:
- Describe the preparation of carboxylic acids.
- Examine chemical reactions of and with carboxylic acids.
Organic functional groups can be converted into other functional groups through reactions. A map of some of the more common reactions to convert functional groups can be found in Section 19.6 – General Reactions of Carbon in Infographic 19.6a.
Preparation of Carboxylic Acids
Oxidation
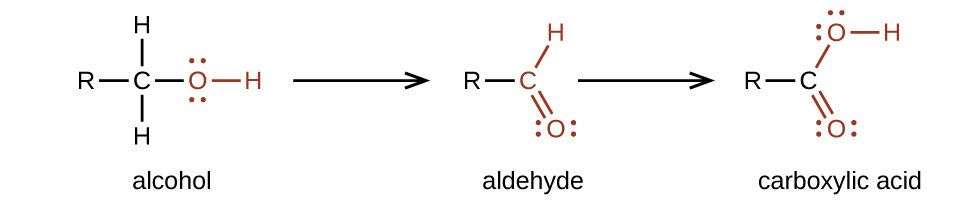
Carboxylic acids are the most polar organic compounds because both functional groups are polar. The hydroxyl (-OH) group is similar to that in alcohols while the carbonyl group (C=O) has similarities to aldehydes and ketones. We prepare carboxylic acids by the oxidation of aldehydes or alcohols whose –OH functional group is located on the carbon atom at the end of the chain of carbon atoms in the alcohol (Figure 25.3a.).
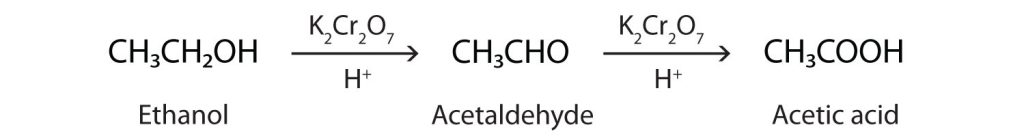
For example, in the presence of an oxidizing agent, ethanol is oxidized to acetaldehyde, which is then oxidized to acetic acid (Figure 25.3b.). This process also occurs in the liver, where enzymes catalyze the oxidation of ethanol to acetic acid using dehydrogenase. Acetic acid can be further oxidized to carbon dioxide and water.
Hydrolysis of Nitriles
Nitriles are organic compounds in which a cyano group (carbon triple bonded to a nitrogen) is attached to a carbon. In Chapter 24, hydrogen cyanide was added to an aldehyde or ketone to form a cyanohydrin. The cyanohydrin contains a nitrile (-C≡N – where ≡ is triple bond). Another method to form a nitrile is shown in Figure 25.3c. Here a primary or secondary alkyl halide will react with sodium cyanide in a substitution reaction to form the alkyl nitrile and sodium halide (Morsch et al, n.d.).

Nitriles can undergo hydrolysis reactions in the presence of an acidic or basic aqueous solution to form carboxylic acids. In the case of acid catalysts, the nitrile becomes pronated (the addition of a proton or hydrogen cation to an atom forming a conjugate acid). In the case of basic catalysts, the hydroxide anion is capable of direct addition to the carbon-nitrogen triple bond. The examples below outline the reactions taking place during hydrolysis of nitriles. Figure 25.3d. shows the basic reaction for nitriles in an acidic catalyst.
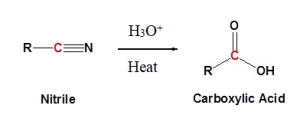
Figure 25.3e. below is a specific example of acidic hydrolysis using cyclopentanecarbonitrile.
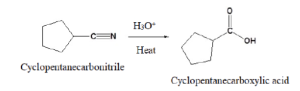
Figure 25.3f., shows the basic hydrolysis reaction of nitriles with an alkaline catalyst.
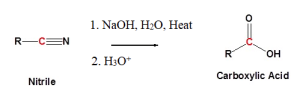
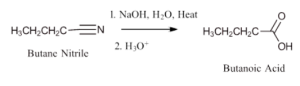
Figure 25.3g. below is a specific example of basic hydrolysis using Butane nitrile.
Reactions of Carboxylic Acids
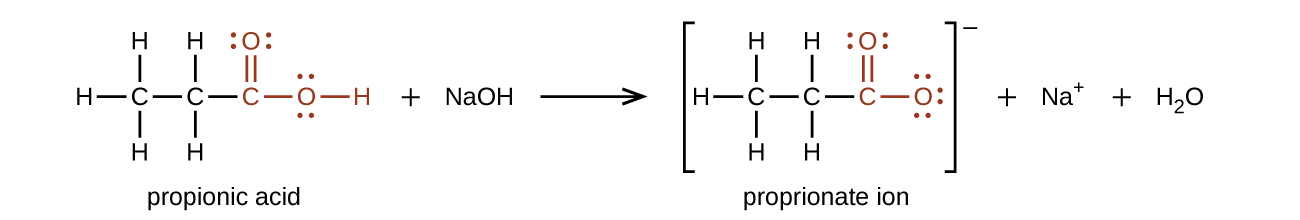
Carboxylic acids are weak acids, meaning they are not 100% ionized in water. Generally, only about 1% of the molecules of a carboxylic acid dissolved in water are ionized at any given time. The remaining molecules are undissociated in solution. They are however considered to be more acidic than most other organic compounds. When carboxylic acids dissociate in water a hydrogen ion is transferred to a water molecule and a carboxylate ion and hydronium ion (H3O+) are formed (Figure 25.3h.). Refer back to Infographic 19.6a showing reactions of organic molecules.
CH3COOH + H2O → CH3COO– + H3O+
Carboxylic Acid + water → Carboxylate Ion + Hydronium Ion
Figure 25.3h. Reaction of a carboxylic acid with water to produce a carboxylate ion and hydronium ion.
Acid-Base Reactions of Carboxylic Acids
Because of the acidic properties of carboxylic acids, they are able to react with bases to form ionic salts. Alkali metal hydroxides and simple amines result in salts with pronounced ionic character that are usually soluble in water. An example of this can be seen in Figure 25.3i. below.
Heavy metals such as silver, mercury and lead form salts with more covalent characteristics which reduces water solubility, particularly for acids composed of four or more carbon atoms in the chain (Figure 25.3j).
RCO2H + AgOH → RCO2δ(-) Agδ(+) + H2O
Figure 25.3j. Reaction of a heavy metal base with a carboxylic acid.
Esterification
Another reaction which takes place with carboxylic acids is esterification. This reaction type is commonly used to convert carboxylic acids to their ester derivatives. In order to produce an ester from an alcohol and a carboxylic acid, we must heat them in the presence of an acid catalyst such as sulfuric acid (Figure 25.3k. and Figure 25.3l.). This reaction will produce a fragrant ester and water. The reaction is reversible and will reach equilibrium with approximately equivalent amounts of reactants and products. Using excess amounts of alcohol and continuously removing a product, can drive the reaction towards the product side as per LeChatelier’s principle.
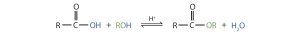
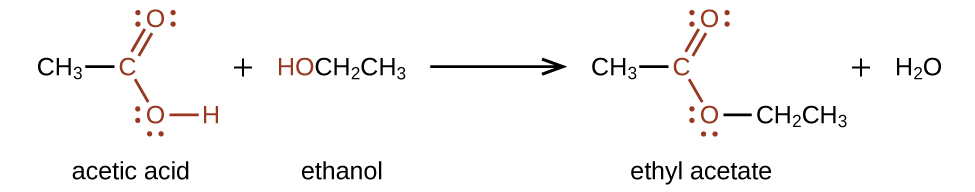
Example 25.3a
Preparation of an ester via esterification uses a carboxylic acid and alcohol, heated in the presence of an acid catalyst (Figure 25.3m.). This reaction is reversible and will reach equilibrium with approximately equal amounts of reactants and products.
Write the esterification of acetic acid with 1-butanol.
Solution:
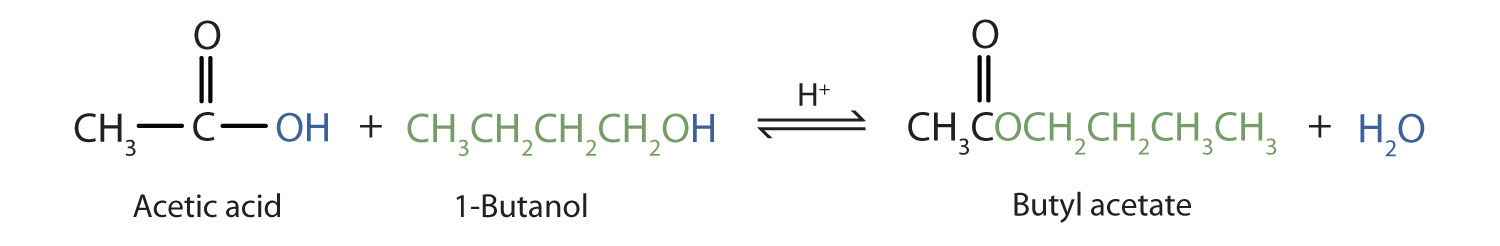
Amide Formation
Similar to esterification, carboxylic acids will react with ammonia to form a primary amide (Figure 25.3n.). When a carboxylic acid reacts with primary or secondary amines, secondary or tertiary amides are produced, respectively (Figure 25.3o.). Tertiary amines do not form amides when reacted with carboxylic acids.
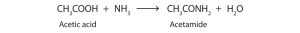

Exercise 25.3a
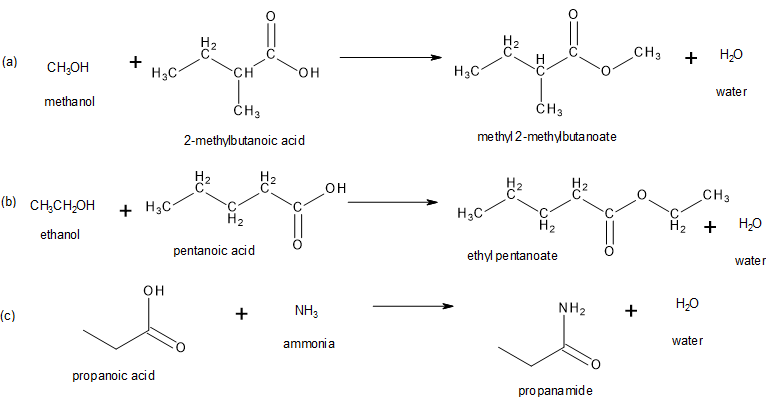
Write the product that results from each of the following reactions.
- methanol + 2-methylbutanoic acid
- ethanol + pentanoic acid
- propanoic acid + ammonia
Check Your Answers:[1]
Exercise and image source: Exercise 25.3a questions and answers are created by Samantha Sullivan Sauer, using images from Biovia Draw, licensed under CC BY-NC 4.0
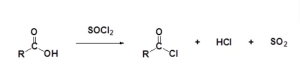
Acid Chloride Formation and Reactions
Carboxylic acids react with thionyl chloride (SOCl2) to form acid chlorides (also known as acyl chlorides) (Figure 25.3p.). During the reaction the hydroxyl group of the carboxylic acid is converted to a chlorosulfite intermediate making it a better leaving group. The chloride anion produced during the reaction acts a nucleophile. Acyl chlorides are extremely reactive, resulting in the chlorine being replaced with something else.
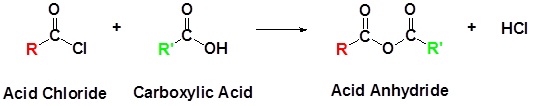
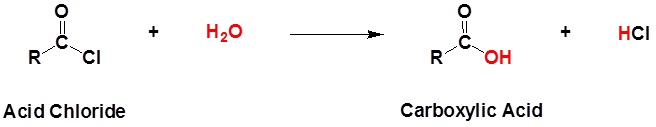
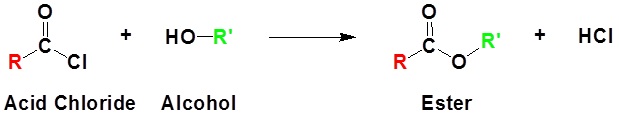
An acid chloride will react with a carboxylic acid to form an acid anhydride (Figure 25.3q.), with water to form a carboxylic acid (Figure 25.3r.), with an alcohol to form an ester (Figure 25.3s.) and with ammonia or an amine to form an amide (Figure 25.3t.).
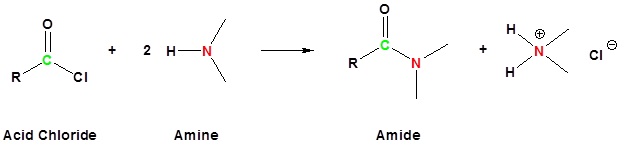
Example 25.3b
Complete each reaction.
(a) 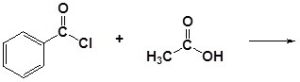
(b) 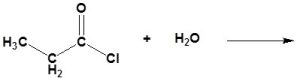
(c) 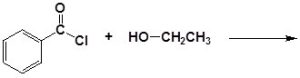
(d) 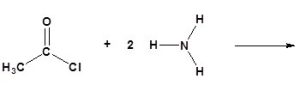
(e) 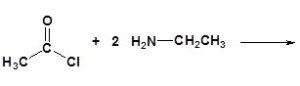
Solutions:
(a) ![]()
(b) ![]()
(c) ![]()
(d) 
(e) ![]()
Example source and images source: UIS: CHE 269 (Morsch and Andrews), CC BY-NC-SA 4.0).
For more advanced understanding of Carboxylic acid structure and reactions check out the videos below.
Watch Crash Course – Organic Chemistry #30 on YouTube (11 min)
Video source: Crash Course – Organic Chemistry. (2021, June 23). Carboxylic Acids: Crash Course Organic Chemistry #30 [Video]. YouTube.
Watch Crash Course – Organic Chemistry #31 on YouTube (12 min)
Video source: Crash Course – Organic Chemistry. (2021, July 28). Carboxylic Acid Derivatives and Hydrolysis Reactions: Crash Course Organic Chemistry #31 [Video]. YouTube.
Attribution & References
Except where otherwise noted, this page is written and adapted by Caryn Fahey and Samantha Sullivan Sauer from
- “15.2: The Formation of Carboxylic Acids” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative authored, remixed, and/or curated by Anonymous via source content that was edited to the style and standards of the LibreTexts platform.
- “18.3 Aldehydes, Ketones, Carboxylic Acids, and Esters” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “20.7: Chemistry of Nitriles” by Steven Farmer, Dietmar Kennepohl, Layne Morsch, William Reusch In Organic Chemistry (Morsch et al.), CC BY-SA 4.0.
- “17.3: Reactions of Carboxylic Acids – Ester and Amide Formation” In Organic Chemistry (OpenStax) by John McMurray, CC BY-NC-SA 4.0. Access for free at Organic Chemistry (OpenStax)
- “Conversion of carboxylic acids to acid chlorides” by Steven Farmer In Supplemental Modules, CC BY-NC-SA 4.0.
- “22.7 Reactions of Acid Chlorides” by Layne Morsch In UIS: CHE 269 (Morsch and Andrews) , CC BY-NC-SA 4.0.
References cited in-text
Farmer, S., Kennepohl, D., Morsch, L., & Reusch, W. (n.d.). Chemistry of Nitriles. In Organic Chemistry (Morsch et al.). CC BY-SA 4.0.
An organic compound containing a cyano group (carbon triple bonded to nitrogen) us attached to a carbon atom. These compounds are colourless solids or liquids with distinctive odours.