20.4 Cycloalkanes
Learning Objectives
By the end of this section, you will be able to:
- Name cycloalkanes given their formulas and write formulas for these compounds given their names.
Cylcoalkanes
The hydrocarbons we have encountered so far have been composed of molecules with open-ended chains of carbon atoms. When a chain contains three or more carbon atoms, the atoms can join to form ring or cyclic structures. The simplest of these cyclic hydrocarbons has the formula C3H6. Each carbon atom has two hydrogen atoms attached (Figure 20.4a.) and is called cyclopropane.
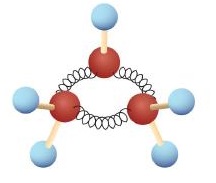
Spotlight on Everyday Chemistry: Cyclopropane (C3H6) as an Anesthetic
With its boiling point of −33°C, cyclopropane is a gas at room temperature. It is also a potent, quick-acting anesthetic with few undesirable side effects in the body. It is no longer used in surgery, however, because it forms explosive mixtures with air at nearly all concentrations. A line structure of cyclopropane is shown in Figure 20.4b.
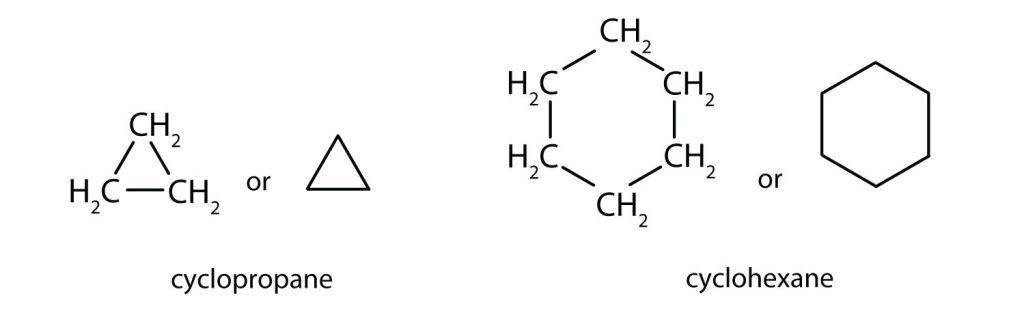
The cycloalkanes—cyclic hydrocarbons with only single bonds—are named by adding the prefix cyclo- to the name of the open-chain compound having the same number of carbon atoms as there are in the ring. Thus the name for the cyclic compound C4H8 is cyclobutane. The carbon atoms in cyclic compounds can be represented by line structure formulas that result in regular geometric figures. Keep in mind, however, that each corner of the geometric figure represents a carbon atom plus as many hydrogen atoms as needed to give each carbon atom four bonds. Figure 20.4 demonstrates the line structure formulas of both cyclopropane and cyclohexane.
Some cyclic compounds have substituent groups attached. Example 20.4a interprets the name of a cycloalkane with a single substituent group.
Draw the structure for each compound.
- cyclopentane
- methylcyclobutane
Solution
- The name cyclopentane indicates a cyclic (cyclo) alkane with five (pent-) carbon atoms. It can be represented as a pentagon.

(Credit: Intro Chem: GOB (V. 1.0). ,CC BY-NC-SA 3.0. )
- The name methylcyclobutane indicates a cyclic alkane with four (but-) carbon atoms in the cyclic part. It can be represented as a square with a CH3 group attached.

(Credit: Intro Chem: GOB (V. 1.0). ,CC BY-NC-SA 3.0.)
Exercise 20.4a
The properties of cyclic hydrocarbons are generally quite similar to those of the corresponding open-chain compounds. So cycloalkanes (with the exception of cyclopropane, which has a highly strained ring) act very much like noncyclic alkanes. Cyclic structures containing five or six carbon atoms, such as cyclopentane and cyclohexane, are particularly stable. We will see later that some carbohydrates (sugars) form five- or six-membered rings in solution.
The cyclopropane ring is strained because the C–C–C angles are 60°, and the preferred (tetrahedral) bond angle is 109.5°. (This strain is readily evident when you try to build a ball-and-stick model of cyclopropane; see Figure 20.4a.) Cyclopentane and cyclohexane rings have little strain because the C–C–C angles are near the preferred angles. Cyclohexane is shown in Figure 20.4b.
Substituted Cycloalkanes
We’ll see numerous instances in future chapters where the chemistry of a given functional group is affected by being in a ring rather than an open chain. Because cyclic molecules are encountered in most pharmaceuticals and in all classes of biomolecules, including proteins, lipids, carbohydrates, and nucleic acids, it’s important to understand the behaviour of cyclic structures.
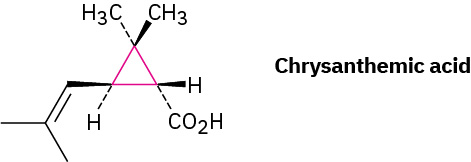
Although we’ve only discussed open-chain compounds up to now, most organic compounds contain rings of carbon atoms. Chrysanthemic acid in Figure 20.4c, for instance, whose esters occur naturally as the active insecticidal constituents of chrysanthemum flowers, contains a three-membered (cyclopropane) ring.
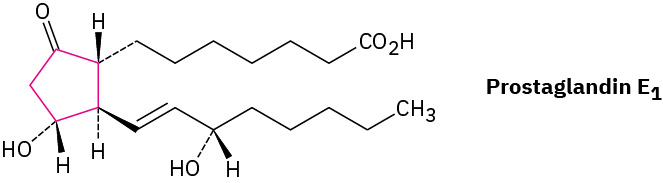
Prostaglandins, potent hormones that control an extraordinary variety of physiological functions in humans, contain a five-membered (cyclopentane) ring. An example of a prostaglandin is in Figure 20.4d.
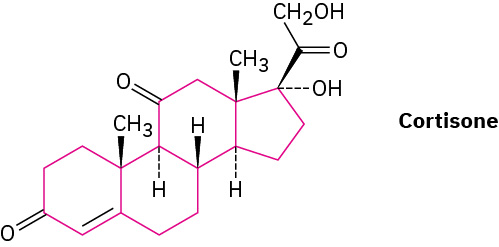
Steroids, such as cortisone, contain four rings joined together—three six-membered (cyclohexane) and one five-membered.
Substituted cycloalkanes are named by rules similar to those we saw for open-chain alkanes. For most compounds, there are only two steps.
- Find the parent. Count the number of carbon atoms in the ring and the number in the largest substituent. If the number of carbon atoms in the ring is equal to or greater than the number in the substituent, the compound is named as an alkyl-substituted cycloalkane. If the number of carbon atoms in the largest substituent is greater than the number in the ring, the compound is named as a cycloalkyl-substituted alkane. Refer to Figure 20.4f. for examples of substituted cycloalkanes.
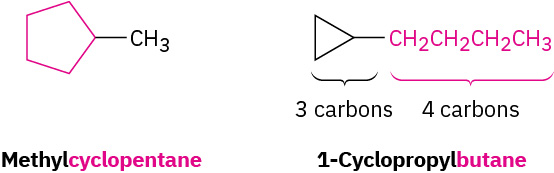
Figure 20.4f. Alkyl substituted cycloalkanes methylcyclopentane and 1-cyclopropyl butane. (credit: Organic Chemistry (OpenStax), CC BY-NC-SA 4.0) - Number the substituents and write the name. For an alkyl- or halo-substituted cycloalkane, choose a point of attachment as carbon 1 and number the substituents on the ring so that the second substituent has as low a number as possible. If ambiguity still exists, number so that the third or fourth substituent has as low a number as possible, until a point of difference is found as shown in the example within Figure 20.4g.
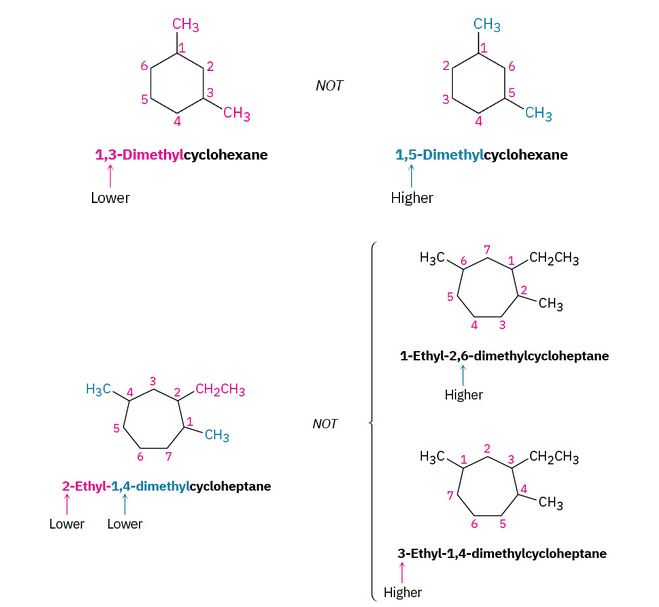
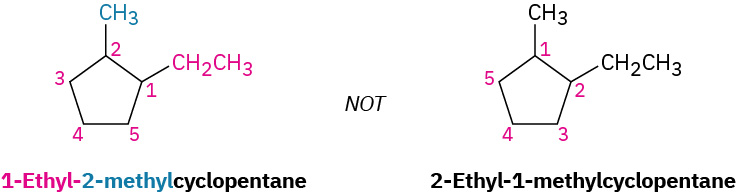
(a) When two or more different alkyl groups are present that could potentially take the same numbers, number them by alphabetical priority, ignoring numerical prefixes such as di- and tri-. An example is demonstrated in Figure 20.4h.
(b) If halogens are present, treat them just like alkyl groups as shown in Figure 20.4i.
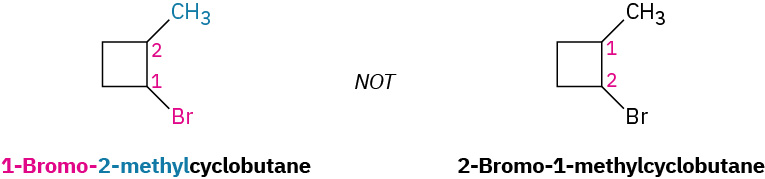
Some additional examples that follow the IUPAC nomenclature system for cycloalkanes are demonstrated in Figure 20.4j.
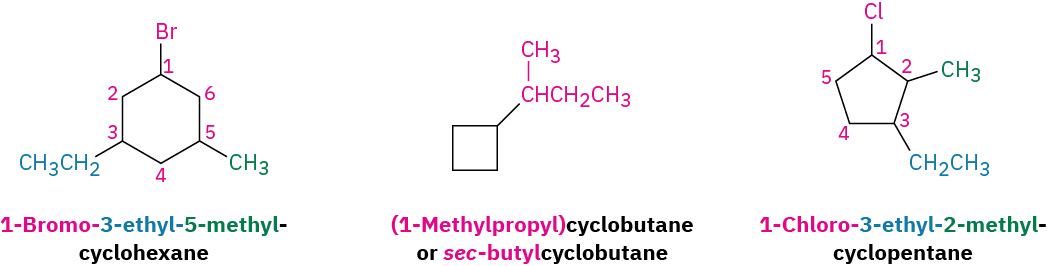
Exercise 20.4b
Name each structure.
Check Your Answers:[2]
Activity source: Exercise 20.4b is created by Samantha Sullivan Sauer, using images from Biovia Draw, licensed under CC BY-NC 4.0
Cis-Trans Isomerism of Cycloalkanes
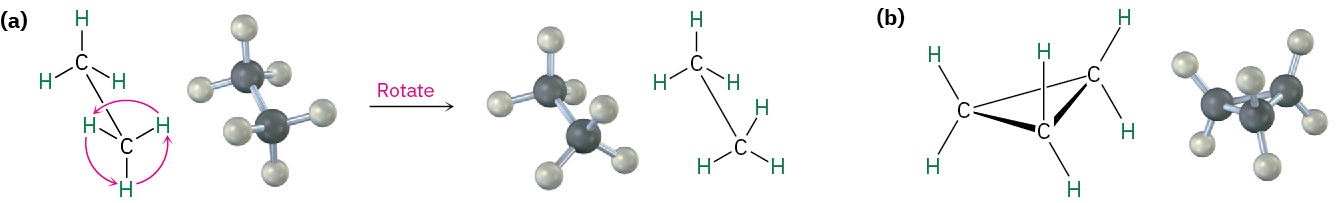
In many respects, the chemistry of cycloalkanes is like that of open-chain alkanes: both are nonpolar and fairly inert. There are, however, some important differences. One difference is that cycloalkanes are less flexible than open-chain alkanes. In contrast with the relatively free rotation around single bonds in open-chain alkanes, there is much less freedom in cycloalkanes. Cyclopropane, for example, must be a rigid, planar molecule because three points (the carbon atoms) define a plane. No bond rotation can take place around a cyclopropane carbon–carbon bond without breaking open the ring as shown in Figure 20.4k.
Larger cycloalkanes have increasing rotational freedom, and very large rings (C25 and up) are so floppy that they are nearly indistinguishable from open-chain alkanes. The common ring sizes (C3–C7), however, are severely restricted in their molecular motions.
Because of their cyclic structures, cycloalkanes have two faces when viewed edge-on, a “top” face and a “bottom” face. As a result, isomerism is possible in substituted cycloalkanes. For example, there are two different 1,2-dimethylcyclopropane isomers, one with the two methyl groups on the same face of the ring and one with the methyl groups on opposite faces as shown in Figure 20.4l. Both isomers are stable compounds, and neither can be converted into the other without breaking and reforming chemical bonds.
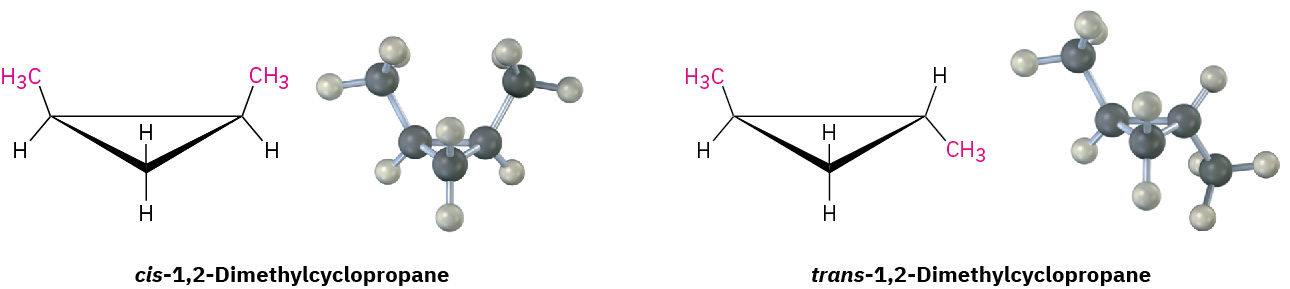
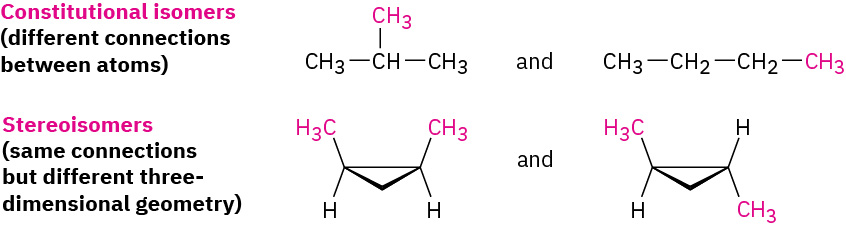
Unlike the constitutional isomers butane and isobutane, which have their atoms connected in a different order, the two 1,2-dimethylcyclopropanes have the same order of connections but differ in the spatial orientation of the atoms. Such compounds, with atoms connected in the same order but differing in three-dimensional orientation, are called stereochemical isomers, or stereoisomers. As we saw previously, the term stereochemistry is used generally to refer to the three-dimensional aspects of structure and reactivity. Figure 20.4m. demonstrates the difference between the types of isomers.
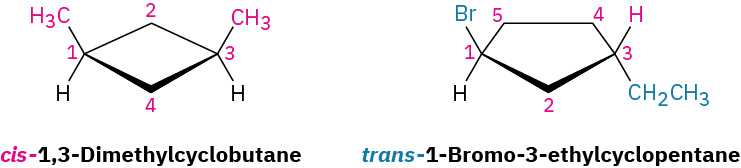
The 1,2-dimethylcyclopropanes are members of a subclass of stereoisomers called cis–trans isomers as shown in Figure 20.4n. The prefixes cis– (Latin “on the same side”) and trans– (Latin “across”) are used to distinguish between them. Cis–trans isomerism is a common occurrence in substituted cycloalkanes and in many cyclic biological molecules.
Example 20.4b
Naming Cycloalkanes
Name the following substances, including the cis– or trans– prefix:
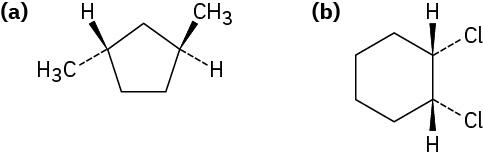
Strategy
In these views, the ring is roughly in the plane of the page, a wedged bond protrudes out of the page, and a dashed bond recedes into the page. Two substituents are cis if they are both out of or both into the page, and they are trans if one is out of and one is into the page.
Solution
(a) trans-1,3-Dimethylcyclopentane
(b) cis-1,2-Dichlorocyclohexane
For a more in-depth look at cycloalkanes, watch the video Cyclohexanes as shown below.
Watch Cyclohexanes: Crash Course Organic Chemistry #7 – YouTube (14 min)
Video source: Crash Course. (2020, July 8). Cyclohexanes: Crash Course Organic Chemistry #7 – YouTube [Video]. YouTube.
Spotlight on Everyday Chemistry: Carp and The Earthy Flavour Geosmin
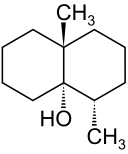
Serving carp is common at Christmas in Europe. Thanks to the cycloalkane compound geosmin (Figure 20.4o), it gives the carp an earthly flavour. For more information see the infographic Compound Interest: The Chemistry Advent Calendar 2023 (compoundchem.com).
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from
- “12.9: Cycloalkanes” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: General, Organic, and Biological (V. 1.0), CC BY-NC-SA 3.0.
- “Ch. 4 Why This Chapter?“, “4.1 Naming Cycloalkanes” and “4.2 Cis–Trans Isomerism in Cycloalkanes” In Organic Chemistry (OpenStax) licensed under CC BY-NC-SA 4.0. Access for free at Organic Chemistry (OpenStax)
-
 b. See the image: Ethylcyclohexane | C8H16 | CID 15504 - PubChem (nih.gov).
Image Source: a. Image by Rhododendronbusch, PDM
b. See the image: Ethylcyclohexane | C8H16 | CID 15504 - PubChem (nih.gov).
Image Source: a. Image by Rhododendronbusch, PDM
↵
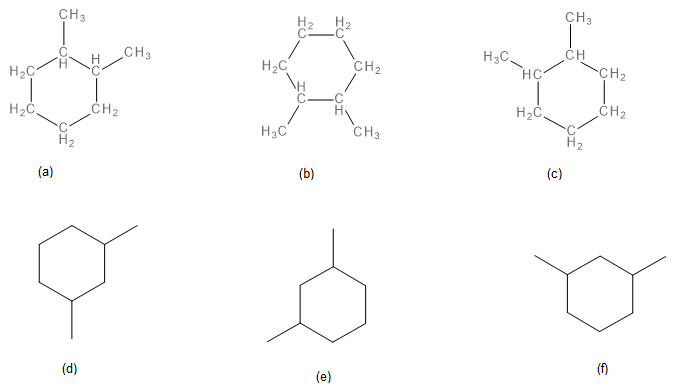
-
Structures a, b, and c are the same. 1,2-dimethylcyclohexane. Structures d, e, and f are the same. 1,3-dimethylcyclohexane. Ring number start at the location that results in the lowest number for all substituents and can proceed in a clockwise or counterclockwise direction.
↵

