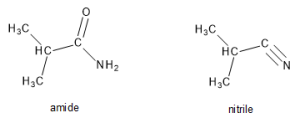
26.6 Chemical Properties of Amines and Amides
Learning Objectives
By the end of this section, you will be able to:
- Identify the typical reaction that amides undergo.
- Identify and describe the substances from which most amines and amides are prepared.
- Describe the preparation procedure for amines and amides.
Organic functional groups can be converted into other functional groups through reactions. A map of some of the more common reactions to convert functional groups can be found in Section 19.6 - General Reactions of Carbon in Infographic 19.6a.
Reactions of Amides
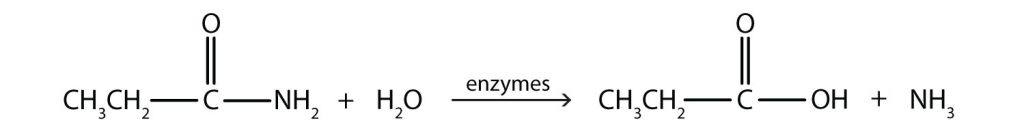
Generally, amides resist hydrolysis in plain water, even after prolonged heating. In the presence of added acid or base, however, hydrolysis proceeds at a moderate rate. In living cells, amide hydrolysis is catalyzed by enzymes. Amide hydrolysis is illustrated in Figure 26.6a.
Example 26.6a
Write the equation for the hydrolysis of each compound.
- butyramide
- benzamide
Solution
-
- The hydrolysis of a simple amide produces an organic acid and ammonia. Butyramide thus yields butyric acid and ammonia.
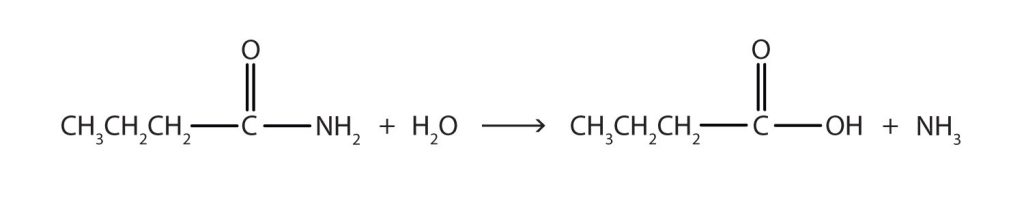
Figure 26.6b. Solution for reaction of butyramide and water.
- The hydrolysis of a simple amide produces an organic acid and ammonia. Butyramide thus yields butyric acid and ammonia.
-
- The hydrolysis of an amide produces an organic acid and ammonia. Benzamide thus yields benzoic acid and ammonia.
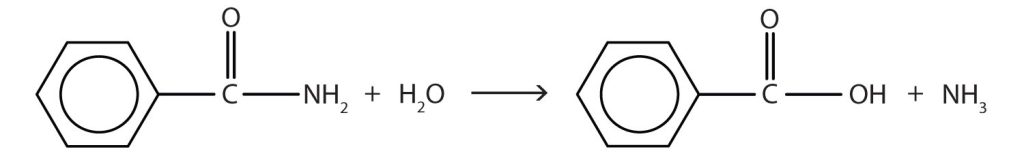
Figure 26.6c. Reaction of benzamide and water.
- The hydrolysis of an amide produces an organic acid and ammonia. Benzamide thus yields benzoic acid and ammonia.
Exercise and Image Source: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0
Exercise 26.6a
What are the products of the hydrolysis of an amide?
Check Your Answers:[1]
Exercise 26.6b
When the amide CH3CH2CH2CH2CONH2 is hydrolyzed in an NaOH solution, the products are CH3CH2CH2CH2COO−Na+ and NH3. What products are obtained when CH3CH2CH2CH2CONH2 is hydrolyzed in an hydrochloric acid solution?
Check Your Answers:[2]
Formation of Amides
Amides can be produced when carboxylic acids react with amines or ammonia in a process called amidation. A water molecule is eliminated from the reaction, and the amide is formed from the remaining pieces of the carboxylic acid and the amine (note the similarity to formation of an ester from a carboxylic acid and an alcohol discussed in the previous section).
When a carboxylic acid reacts with ammonia (NH3) a primary amide is formed. But the reaction is very slow at room temperature. Water molecules are split out, and a bond is formed between the nitrogen atom and the carbonyl carbon atom (Figure 26.6d.).
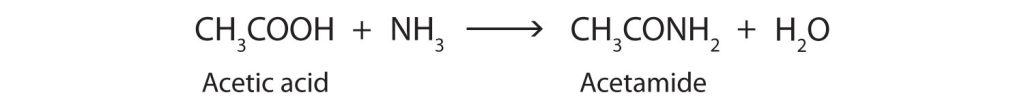
When a carboxylic acid reacts with primary or secondary amines, secondary or tertiary amides are produced, respectively (Figure 26.6e.).
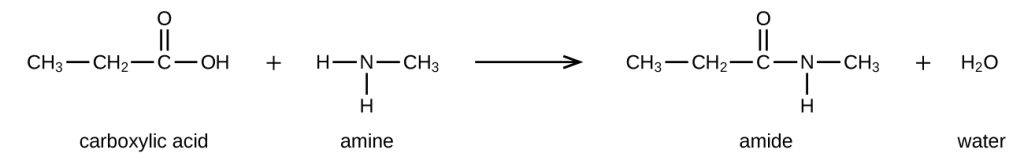
Tertiary amines do not have a hydrogen attached to the nitrogen and therefore do not form amides when mixed with carboxylic acids. However, an acid-base reaction does occur with the amine accepting a proton (acts as a base) and the carboxylic acid donating a proton. In this case the ammonium and carboxylate salts are formed.
In living cells, amide formation is catalyzed by enzymes. Proteins, which make up all enzymes, are polyamides; they are formed by joining amino acids into long chains. In proteins, the amide functional group is called a peptide bond.
Carboxylic acids will react with alcohols and amines following a similar pattern. In both cases, the –OH group of the carboxylic acid will be replaced by a different group to form either an ester or an amide, with water formed as a by-product. When the reaction involves an alcohol, the –OH of the acid is replaced by the –OR' of the alcohol (Figure 26.6f.). When the reaction involves an amine, the –OH of the acid is replaced by the –NH2, or –NHR', or –NR'2 of the amine (Figure 26.6g.).
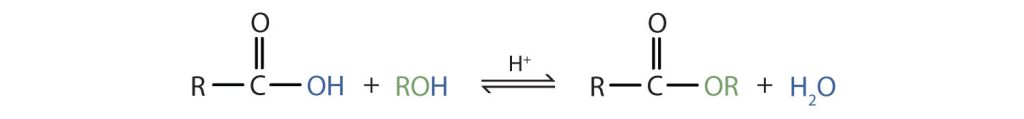

Amides can also be prepared from the reaction of an acid chloride with ammonia. Refer to Section 25.3 Formation and Reactions of Carboxylic Acids where the formation of acid chlorides is discussed as well as their reaction with ammonia.
Formation of Amines
Reduction of Amides
Like other carboxylic acid derivatives, amides can be reduced by LiAlH4. The product of the reduction, however, is an amine rather than an alcohol (Figure 26.6h.). The net effect of an amide reduction is thus the conversion of the amide carbonyl group into a methylene group (C=O⟶CH2). This kind of reaction is specific to amides and does not occur with other carboxylic acid derivatives.
Example 26.6b
Synthesizing an Amine from an Amide
How could you prepare N-ethylaniline by reduction of an amide with LiAlH4?
Strategy
Reduction of an amide with LiAlH4 yields an amine. To find the starting material for synthesis of N-ethylaniline, look for a CH2 position next to the nitrogen atom and replace that CH2 by C═O. In this case, the amide is N-phenylacetamide.
Solution
Reduction of Nitriles
Using lithium aluminum hydride (LiAlH4), a nitrile can be reduced to a primary amine (Figure 26.6k.). The process to predict the product of a hydride reduction is shown in Figure 26.6l. (Farmer et al., n.d.).
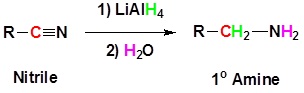
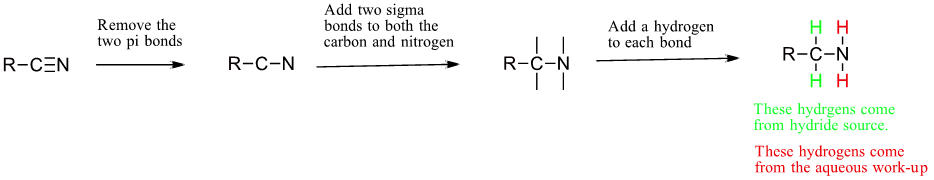
Example 26.6c
Write the product from this reaction.
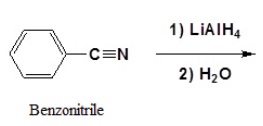
Solution:
Following the process outlined in Figure 26.6l, the answer is
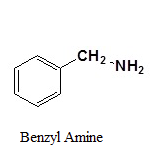
Source: Example 26.6c is adapted from "20.7: Chemistry of Nitriles" by Steven Farmer, Dietmar Kennepohl, Layne Morsch, William Reusch In Organic Chemistry (Morsch et al.), CC BY-SA 4.0. / Image cut in two.
The formation of nitriles (R-C≡N) can be found in Section 25.3 - Formation and Reactions of Carboxylic Acids.
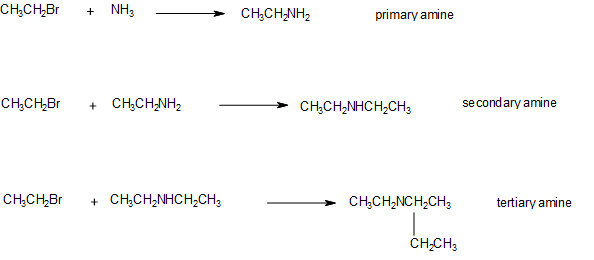
Alkylation of Ammonia
Another way to produce an amine is to react an alkyl halide (also called haloalkane) with ammonia. This reaction substitutes one hydrogen of the ammonia with the alkyl portion of the alkyl halide. It can be completed with ammonia to produce a 1o amine or with a 1o amine to produce a 2o amine or with a 2o amine to produce a 3o amine.
Exercise 26.6c
Write the structures of the amide and the nitrile that when reduced produce this amine.
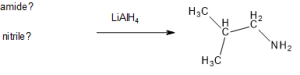
Check Your Answers:[3]
Source: Exercise 26.6c created by Samantha Sullivan Sauer, images drawn using Biovia Draw, CC BY-NC 4.0
Attribution & References
Except where otherwise noted, this page is written and adapted by Caryn Fahey and Samantha Sullivan Sauer from
- "15.16: Chemical Properties of Amides- Hydrolysis" In Basics of General, Organic, and Biological Chemistry (Ball et al.)by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- "20.7: Chemistry of Nitriles" by Steven Farmer, Dietmar Kennepohl, Layne Morsch, William Reusch In Organic Chemistry (Morsch et al.), CC BY-SA 4.0.
- "17.3: Reactions of Carboxylic Acids - Ester and Amide Formation" In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0
- "21.7 Chemistry of Amides" In Organic Chemistry (OpenStax) by John McMurry, licensed under CC BY-NC-SA 4.0. Access for free at Organic Chemistry (OpenStax).
- "Reaction of Alkyl Halides with Ammonia" by Jim Clark In Supplemental Modules, CC BY-NC 4.0
References cited in-text
Farmer, S., Kennepohl, D., Morsch, L., & Reusch, W. (n.d.). 20.7: Chemistry of Nitriles. In Organic Chemistry (Morsch et al.). LibreTexts. CC BY-SA 4.0.
the reaction in which an amide group replaces the hydrogen atom on the amino group.