Chapter 23 – Review
23.1 Alcohols – Structure, Naming and Classification
- Is isobutyl alcohol primary, secondary, or tertiary? Explain. Check answer [1]
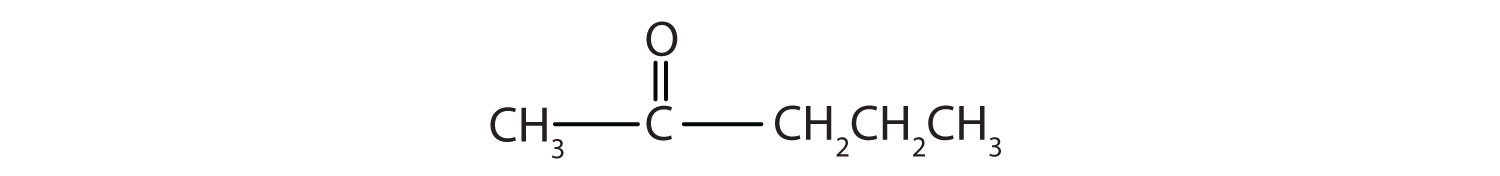
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.) - What is the longest continuous chain (LCC) in 2-ethyl-1-hexanol? What is taken as the LCC in naming the compound? Explain. Check answer[2]
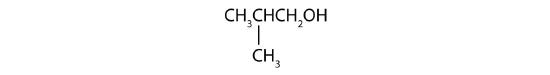
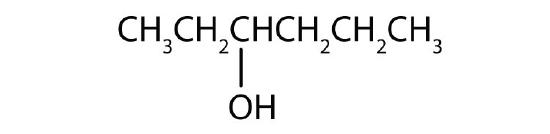
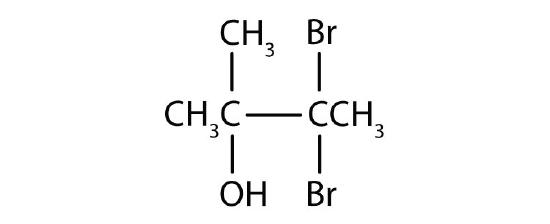
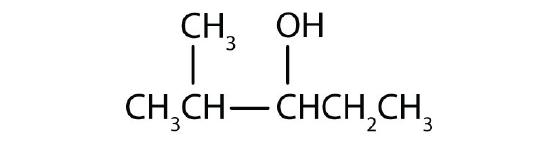
- Name each alcohol and classify it as primary, secondary, or tertiary. Check answer[3]
- CH3CH2CH2CH2CH2CH2OH
-
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.) -
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.)
-
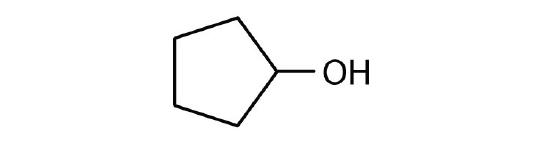
Name each alcohol and classify it as primary, secondary, or tertiary.
-
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.) -
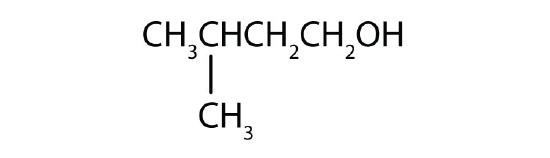
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.) -
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.)
-
- Draw the structure for each alcohol. a. 3-hexanol b. 3,3-dimethyl-2-butanol c. cyclobutanol Check answer[4]
- Draw the structure for each alcohol.
- cyclopentanol
- 4-methyl-2-hexanol
- 4,5-dimethyl-3-heptanol
- What is a glycol? Check answer[5]
- Why is ethylene glycol so much more toxic to humans than propylene glycol?
- Draw the structure for each compound. Check answer[6]
a. 1,5-pentanediol
b. propylene glycol - Draw the structure for each compound.
- 1,3-hexanediol
- glycerol
23.2 Physical Properties of Alcohols
- Why is ethanol more soluble in water than 1-hexanol? Check answer[7]
- Why does 1-butanol have a lower boiling point than 1-hexanol? Check answer[8]
- Arrange these alcohols in order of increasing boiling point: ethanol, methanol, and 1-propanol. Check answer[9]
- Which has the higher boiling point —butane or 1-propanol?
- Arrange these alcohols in order of increasing solubility in water: 1-butanol, methanol, and 1-octanol. Check answer[10]
- Arrange these compounds in order of increasing solubility in water: 1-butanol, ethanol, and pentane.
- Ethanol is used as a solvent for some drugs that are not soluble in water. Why is methanol not used in medicines?
- How does the boiling point and solubility change if more OH groups are included in a molecule? Why?
23.3 Formation of Alcohols
- From what alkene is ethanol made? Draw its condensed structural formula. Check answer[11]
- Can methanol be made from an alkene? Explain.
- When water is added to ethylene in the presence of an acid catalyst, only one product—ethanol—is possible. However, when water is added to propylene, two products are possible—1-propanol and 2-propanol—but only 2-propanol is formed. In 1870, the Russian chemist Vladimir V. Markovnikov proposed a rule to predict the products of such reactions: Considering water to be HOH, the hydrogen atom of water goes on the carbon atom (of the two involved in the double bond) that has the most hydrogen atoms already bonded to it. The OH group goes on the carbon atom with fewer hydrogen atoms. Use Markovnikov’s rule to predict the product of the addition of water to each compound. Check answer[12]
- 2-methylpropene
- 1-butene
- 2-methyl-1-pentene
- 2-methyl-2-pentene
- Alcohols can be made from alkyl halides, aldehydes and ketones, and esters. Provide a description or example of each reaction.
23.4 Reactions of Alcohols
-
Name the three major types of chemical reactions of alcohols. Check answer[13]
-
Why do tertiary alcohols not undergo oxidation? Can a tertiary alcohol undergo dehydration?
-
Draw the structure of the product for each reaction.
-
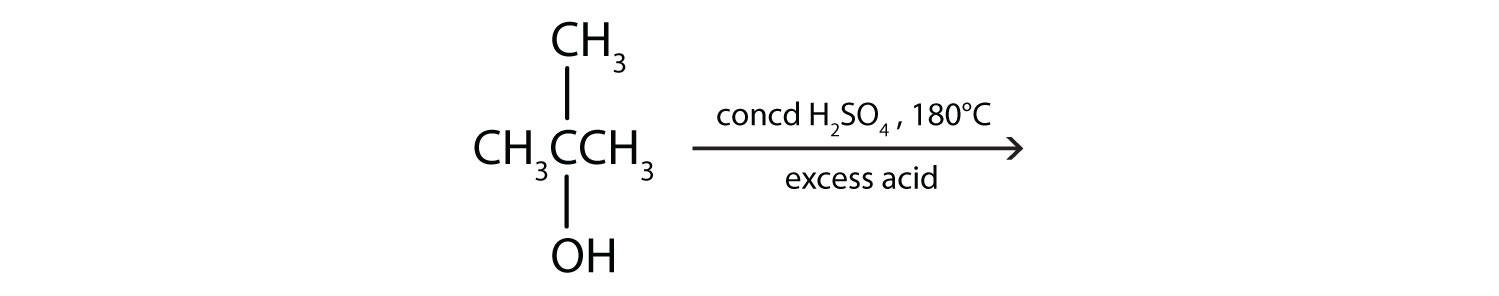
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0). -
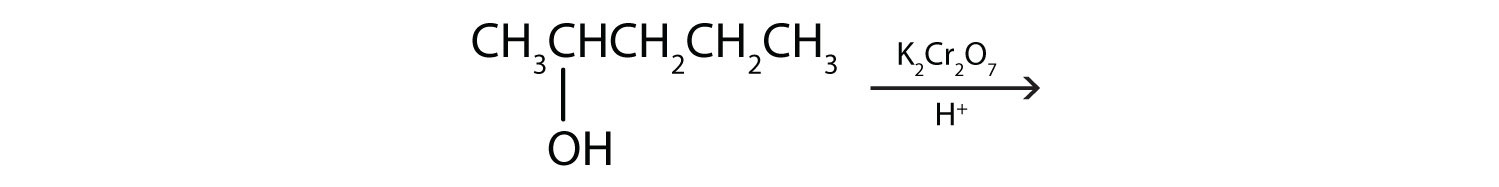
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0). Check answer[14]
-
-
Write an equation for the dehydration of 2-propanol to yield each compound type. Check answer[15]
- an alkene
- an ether
-
Draw the structure of the alkene formed by the dehydration of cyclohexanol.
Classify each conversion as oxidation, dehydration, or hydration (only the organic starting material and product are shown): Check answer[16]
- CH3OH → HCHO
- CH3CHOHCH3 → CH3CH=CH2
- CH2=CHCH2CH3 → CH3CHOHCH2CH3
-
Classify each conversion as oxidation, dehydration, or hydration (only the organic starting material and product are shown.):
- CH3CHOHCH3 → CH3COCH3
- HOOCCH=CHCOOH → HOOCCH2CHOHCOOH
- 2 CH3OH → CH3OCH3
- What does a positive result from the Tollen’s test, Fehling test and Benedict test look like? Check answer[17]
- For each compound, identify if the test result will be positive or negative given the listed test.
- 2-propanol – Tollen’s test
- 2-methyl-2-pentanol – Benedict Test
- cyclopentanol – Fehling Test
- Draw the major product from the dehydration of each compound (assume alkene formation).
- 2-methyl-2-pentanol
- 2-chlorocyclopentanol
- 2-methyl-4-octanol
- Draw the product of esterification of ethanol and ethanoic acid.
- How do phenols differ from alcohols in terms of structure and properties? Check answer[18]
- How do phenols differ in properties from aromatic hydrocarbons? Check answer[19]
- Name each compound. Check answer[20]
-
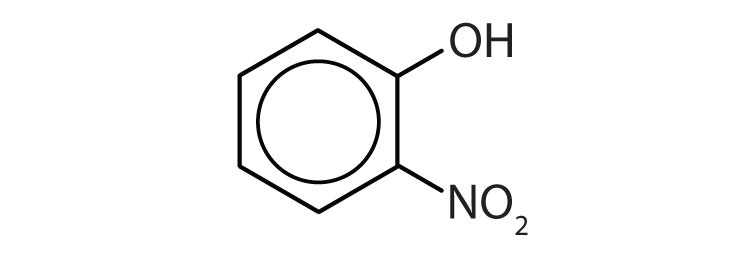
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0). -
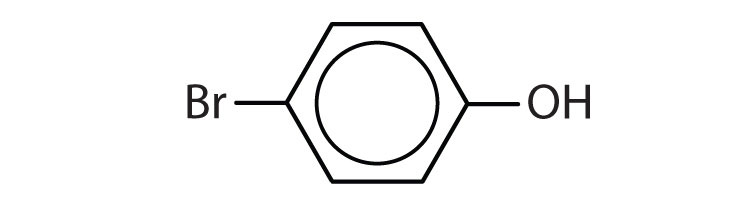
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0).
-
-
Name each compound.
-
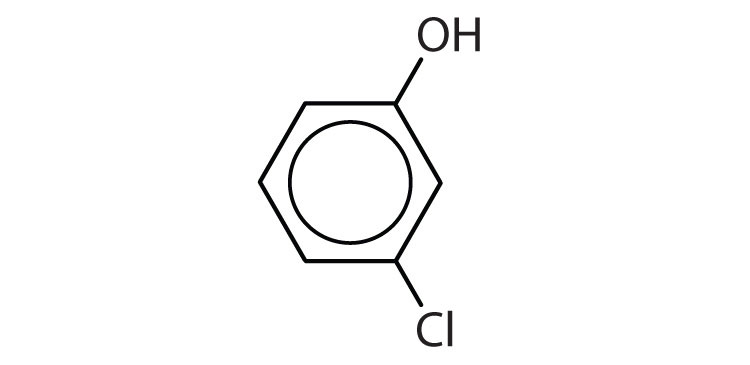
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0). -
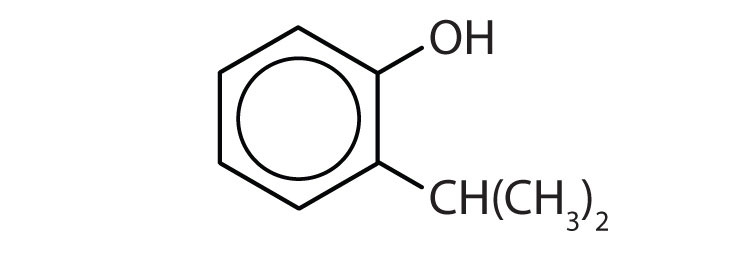
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0).
-
- Draw the structure for each compound. Check answer[21]
a) m-iodophenol
b) p-methylphenol (p-cresol) - Draw the structure for each compound.
- 2,4,6-trinitrophenol (picric acid)
- 3,5-diethylphenol
23.6 Ethers
-
Identify each compound as an alcohol, a phenol, or an ether. Classify any alcohols as primary (1°), secondary (2°), or tertiary (3°).
- CH3CH2CH2OH
-
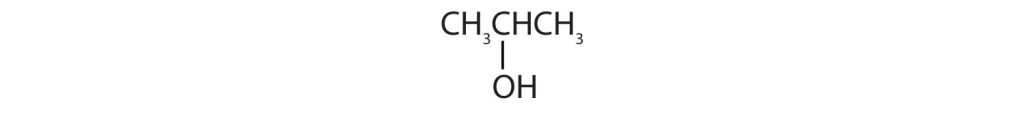
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.) -
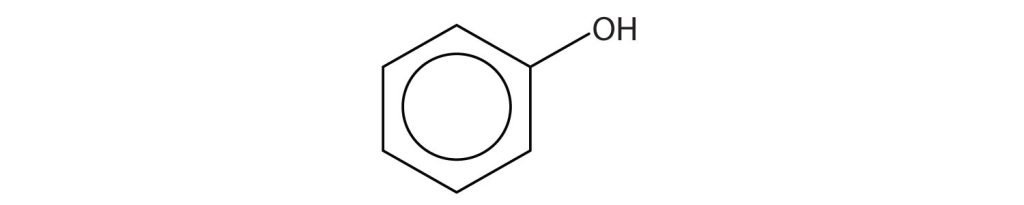
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.) -
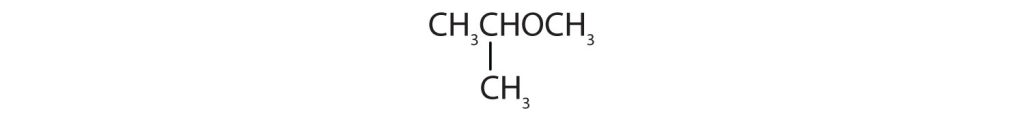
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.)
- Identify each compound as an alcohol, a phenol, or an ether. Classify any alcohols as primary, secondary, or tertiary. Check answer[22]
- CH3CH2OCH2CH3
-
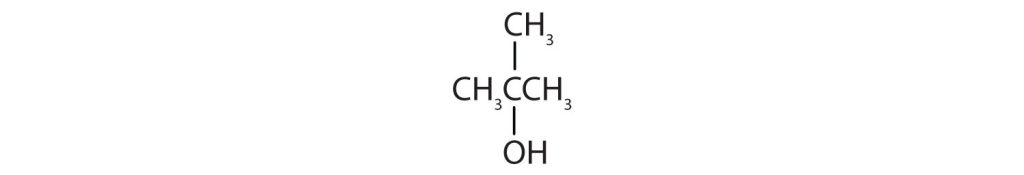
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.) -
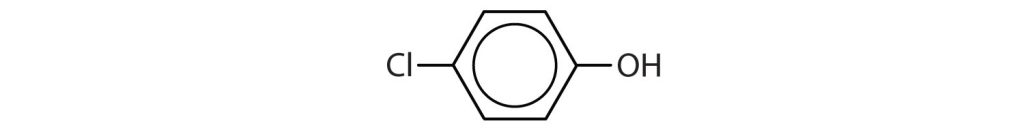
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.) -
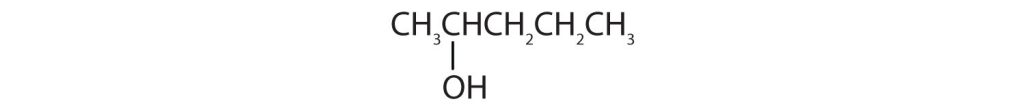
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0.)
- Why does ethoxyethane (CH3CH2OCH2CH3) have a much lower boiling point than 1-butanol (CH3CH2CH2CH2OH)? Check answer[23]
- Which is more soluble in water—methoxyethane (CH3CH2OCH3) or 1-butanol (CH3CH2CH2CH2OH)? Explain. Check answer[24]
- How can ethanol give two different products when heated with sulfuric acid? Name these products. Check answer[25]
- Which of these ethers is isomeric with ethanol—CH3CH2OCH2CH3, CH3OCH2CH3, or CH3OCH3?
- Name each compound. Check answer[26]
- CH3OCH2CH2CH3
-
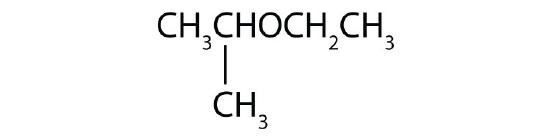
(credit: Intro Chem: GOB, CC BY-NC-SA 3.0).
- Name each compound.
- CH3CH2CH2CH2OCH3
- CH3CH2OCH2CH2CH3
- Draw the structure for each compound. Check answer[27]
-
- methoxyethane
- ethoxytert-butane
-
23.7 Thiols
- What is the functional group of a thiol? Write the condensed structural formula for ethanethiol (ethyl mercaptan). Check answer[28]
- What is the functional group of a disulfide? Write the condensed structural formula for dipropyl disulfide. Check answer[29]
- A common natural gas odourant is tert-butyl mercaptan. What is its condensed structural formula? Check answer[30]
- Write the equation for the oxidation of ethanethiol to diethyl disulfide.
Links to Enhanced Learning
Create your own organic nomenclature quiz to identify, name and draw alcohols and ethers using Organic Nomenclature (orgchem101.com). You can customize the types of questions you receive and get instant feedback.
Attribution & References
Except where otherwise noted, this page (including images in solutions) is written and adapted by David Wegman and Samantha Sullivan Sauer from
- “18.2 Alcohols and Ethers” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “ 14.E: Organic Compounds of Oxygen (Exercises) “, in Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- “14.8: Thiols and Disulfides” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0.
- Images in solutions are from the original source, except:
- 23.1 Question 5: Intro Chem: GOB, CC BY-NC-SA 3.0.
- 23.1 Question 9b: Intro Chem: GOB, CC BY-NC-SA 3.0
- 23.4 Question 3: Intro Chem: GOB, CC BY-NC-SA 3.0
- 23.5 Question 5: Intro Chem: GOB, CC BY-NC-SA 3.0
- 23.6 Question 9b: Intro Chem: GOB, CC BY-NC-SA 3.0
- primary; the carbon atom bearing the OH group is attached to only one other carbon atom ↵
- 7 carbon atoms; the 6-atom chain includes the carbon atom bearing the OH group ↵
- a) 1-hexanol; primary b) 3-hexanol; secondary c) 3,3-dibromo-2-methyl-2-butanol; tertiary ↵
- a)
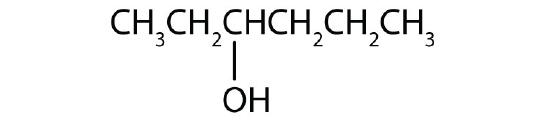 b)
b)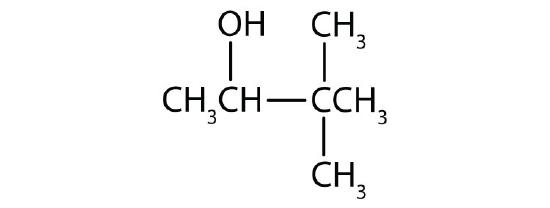 c)
c) ↵
↵ - an alcohol with two OH groups on adjacent carbon atoms ↵
-
a. HOCH2CH2CH2CH2CH2OH
b.
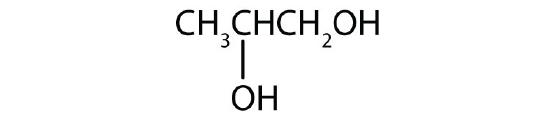 ↵
↵ - Ethanol has an OH group and only 2 carbon atoms; 1-hexanol has one OH group for 6 carbon atoms and is thus more like a (nonpolar) hydrocarbon than ethanol is. ↵
- The molar mass of 1-hexanol is greater than that of 1-butanol. ↵
- methanol < ethanol < 1-propanol ↵
- 1-octanol < 1-butanol < methanol ↵
- ethylene; CH2=CH2 ↵
- a. 2-methyl-2-propanol, b. 2-butanol, c. 2-methyl-2-pentanol, d. 2-methyl-2-pentanol ↵
- dehydration, oxidation, and esterification ↵
-
-
a. CH3CHOHCH3 → CH3COCH3 + H2O (under conditions of conc H2SO4 180∘C excess acid)
b. 2 CH3CHOHCH3 → (CH3)2CHOCH(CH3)2 + H2O (under conditions of conc H2SO4 140∘C, excess alcohol)
- a. oxidation, b. dehydration, c. hydration ↵
- Tollens - silver mirror finish, Fehling - red precipitate, Benedict - red precipitate ↵
- Phenols have an OH group attached directly to an aromatic ring. Phenols are weakly acidic. ↵
- Phenols have an OH group and are somewhat soluble in water. ↵
- 1) o-nitrophenol 2) p-bromophenol ↵
-
a)
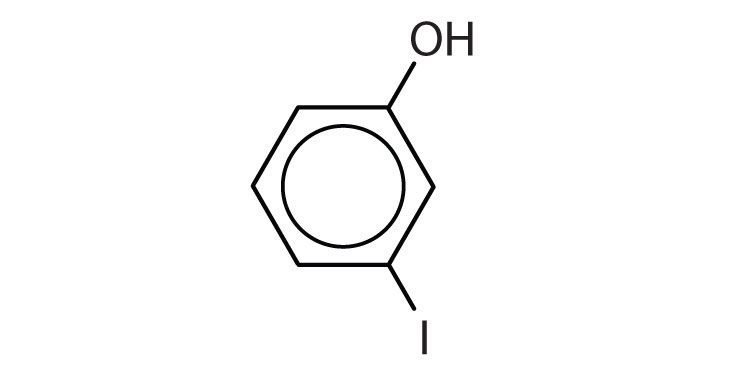 b)
b)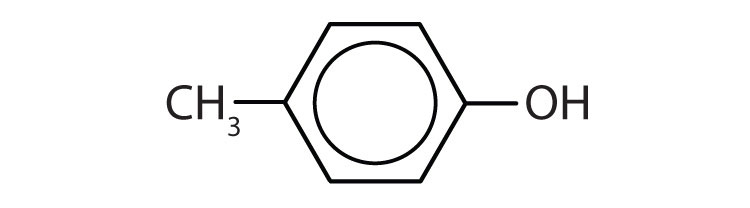 ↵
↵ - a. ether b. tertiary alcohol c. phenol d. secondary alcohol ↵
- Ethoxyethane has no intermolecular hydrogen bonding because there is no OH group; 1-butanol has an OH and engages in intermolecular hydrogen bonding. ↵
- methoxyethane (three carbon atoms, one oxygen atom) is more soluble in water than 1-butanol (four carbon atoms, one oxygen atom), even though both can engage in hydrogen bonding with water. ↵
- Intramolecular (both the H and the OH come from the same molecule) dehydration gives ethene; intermolecular (the H comes from one molecule and the OH comes from another molecule) dehydration gives ethoxyethane. ↵
- 1) methoxypropane 2) ethoxyisopropane ↵
- 1) CH3OCH2CH3 2)
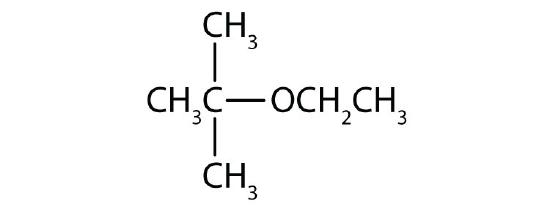 ↵
↵ - SH; CH3CH2SH ↵
- –S–S–; CH3CH2CH2SSCH2CH2CH3 ↵
- (CH3)3CSH ↵