Chapter 20 – Review
20.1 Characteristics of Alkanes
- Without referring to a table, predict which has a higher boiling point—hexane or octane. Explain. Check answer[1]
- If 25 mL of hexane were added to 100 mL of water in a beaker, which of the following would you expect to happen? Explain.
- Hexane would dissolve in water.
- Hexane would not dissolve in water and would float on top. Check answer [2]
- Hexane would not dissolve in water and would sink to the bottom of the container.
- Without referring to a table or other reference, predict which member of each pair has the higher boiling point.
- For which member of each pair is hexane a good solvent?
- pentane or water
- sodium chloride or soybean oil
- Why are alkanes sometimes called paraffins? Check answer [5]
20.2 Alkane Formulas
-
- Write the condensed structural formula for each structural formula.
-
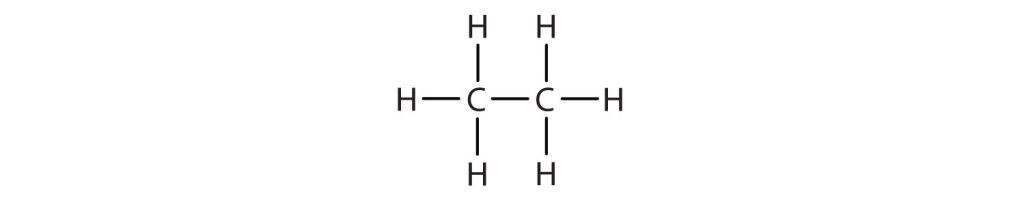
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). Check answer[6]
-
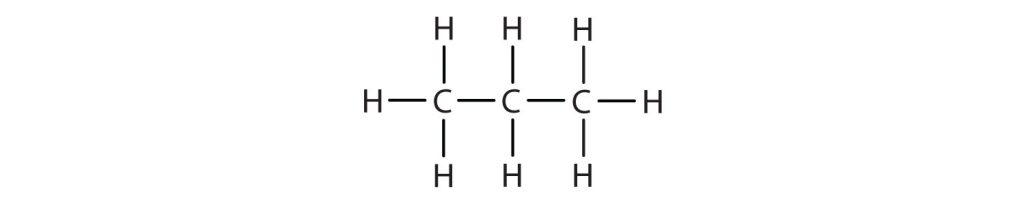
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). Check answer[7]
-
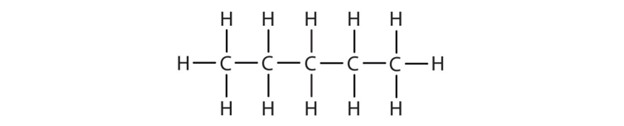
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). Check answer[8]
-
- A condensed structural formula for isohexane can be written as (CH3)2CHCH2CH2CH3. Draw the line-angle formula for isohexane.
- Draw a line-angle formula for the compound CH3CH2CH(CH3)CH2CH2CH3. Check answer[9]
- Give the structural formula for the compound represented by this line-angle formula:
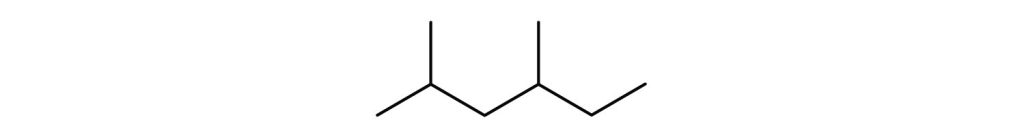
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0).
- Write the condensed structural formula for each structural formula.
20.3 Isomers of Alkanes and IUPAC Nomenclature
- Briefly identify the important distinctions between a straight-chain alkane and a branched-chain alkane. Check answer [10]
- How are butane and isobutane related? How do they differ?
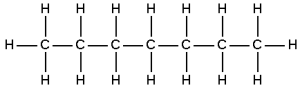
- Name each compound.
-
(Credit: Photo by Bangin, Public Domain) Check answer[11]
-
(Credit: Photo by Magnus Manske , Public Domain) Check answer[12]
-
- Write the structural formula for each compound.
- hexane
- octane
-
Indicate whether the structures in each set represent the same compound or isomers.
-
CH3CH2CH2CH3 and
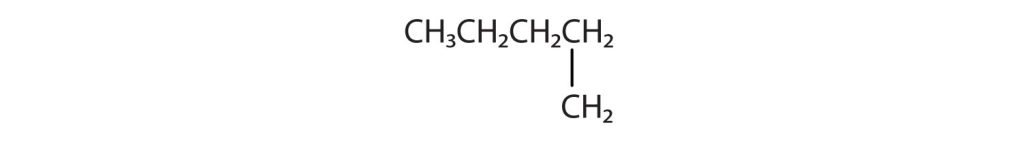 Check answer[13](credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0).
Check answer[13](credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). -
CH3CH2CH2CH2CH3 and
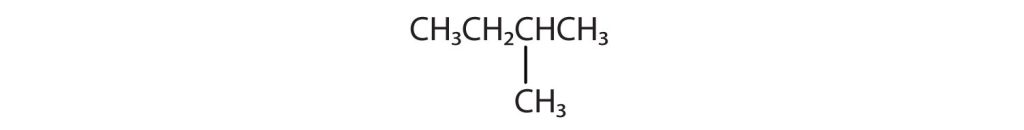
Check answer[14](Credit: Introduction to Chemistry: General, Organic, and Biological (V. 1.0). ,CC BY-NC-SA 3.0.)
-
- Briefly identify the important distinctions between an alkane and an alkyl group. Check answer[15]
- How many carbon atoms are present in each molecule?
- 2-methylbutane
- 3-ethylpentane
- How many carbon atoms are present in each molecule?
- Draw the structure for each compound.
- 3-methylpentane
- 2,2,5-trimethylhexane
- 4-ethyl-3-methyloctane
- Draw the structure for each compound.
- Name each compound according to the IUPAC system.
-
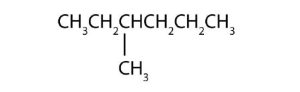
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). -
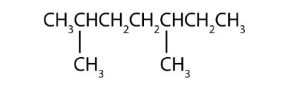
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0).
-
- Name each compound according to the IUPAC system.
-
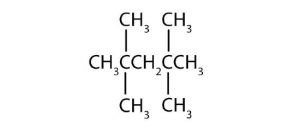
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). Check answer[21]
-
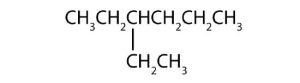
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0).
-
- What is a substituent? How is the location of a substituent indicated in the IUPAC system?
- Briefly identify the important distinctions between a common name and an IUPAC name. Check answer[22]
- Draw the structures for the five isomeric hexanes (C6H14). Name each by the IUPAC system. Check answer[23]
20.4 Cycloalkanes
- What is the molecular formula of cyclooctane? Check answer[24]
- What is the IUPAC name for this compound? Check answer[25]
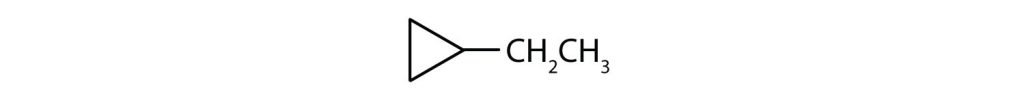
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). -
Draw the structure for each compound.
- Draw the structure for each compound.
- methylcyclohexane
- butylcyclobutane
- Cycloalkyl groups can be derived from cycloalkanes in the same way that alkyl groups are derived from alkanes. These groups are named as cyclopropyl, cyclobutyl, and so on. Name each cycloalkyl halide.
-
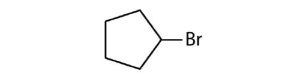
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). Check answer[28]
-
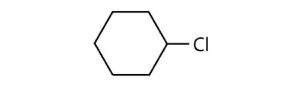
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). Check answer[29]
-
- Halogenated cycloalkanes can be named by the IUPAC system. As with alkyl derivatives, monosubstituted derivatives need no number to indicate the position of the halogen. To name disubstituted derivatives, the carbon atoms are numbered starting at the position of one substituent (C1) and proceeding to the second substituted atom by the shortest route. Name each compound.
-
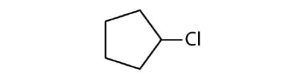
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). edited by Ball et al., CC BY-NC-SA 3.0.) -
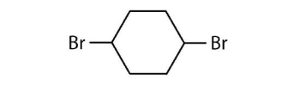
(credit: Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0). edited by Ball et al., CC BY-NC-SA 3.0.)
20.5 Halogenated Alkanes
- What is the IUPAC name for the HFC that has the formula CH2FCF3? (Hint: you must use a number to indicate the location of each substituent F atom.) Check answer [30]
- What is the IUPAC name for the HCFC that has the formula CHCl2CF3? Check answer[31]
- Write the condensed structural formula for each compound.
- Write the condensed structural formula for each compound.
- ethyl bromide
- carbon tetrachloride
- Write the condensed structural formulas for the two isomers that have the molecular formula C3H7Br. Give the common name and the IUPAC name of each. Check answer [34]
- Write the condensed structural formulas for the four isomers that have the molecular formula C4H9Br. Give the IUPAC name of each.
- What is a CFC? How are CFCs involved in the destruction of the ozone layer? Check answer [35]
- Explain why each compound is less destructive to the ozone layer than are CFCs.
- fluorocarbons
- HCFCs
20.6 Reactions of Alkanes
- Which halogen reacts most readily with alkanes? Which reacts least readily? Check answer [36]
- Why do alkanes usually not react with ionic compounds such as most laboratory acids, bases, oxidizing agents, or reducing agents? Check answer[37]
- Write an equation for the complete combustion of methane (CH4), the main component of natural gas.
- What is the most important reaction of alkanes?
- Name some substances other than oxygen that react readily with alkanes.
- Write equations for the complete combustion of each compound.
Links to Enhanced Learning
Attribution & References
Except where otherwise noted, this page (including images in solutions) is adapted by Adrienne Richards from
- “12.E: Organic Chemistry- Alkanes and Halogenated Hydrocarbons (Exercises)” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- Images for solutions to questions 20.2 – 3, 20.3 – 7, and 20.4 – 3 are from Intro Chem: GOB (V. 1.0). , CC BY-NC-SA 3.0.
- octane because of its greater molar mass ↵
- b; hexane is insoluble in water and less dense than water. ↵
- pentane ↵
- nonane ↵
- Alkanes do not react with many common chemicals. They are sometimes called paraffins, from the Latin parum affinis, meaning “little affinity.” ↵
- CH3CH3 ↵
- CH3CH2CH3 ↵
- CH3CH2CH2CH2CH3 ↵
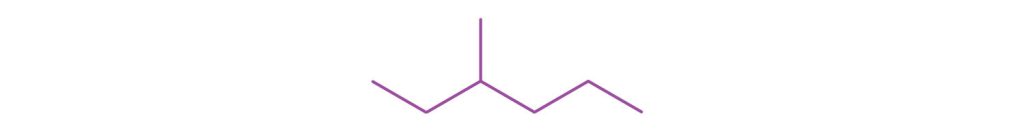 ↵
↵- Straight-chain alkanes and branched-chain alkanes have different properties as well as different structures. ↵
- pentane ↵
- heptane ↵
- no ↵
- yes ↵
- An alkane is a molecule; an alkyl group is not an independent molecule but rather a part of a molecule that we consider as a unit. ↵
- 6 ↵
- 10 ↵
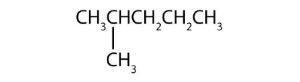 ↵
↵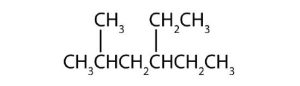 ↵
↵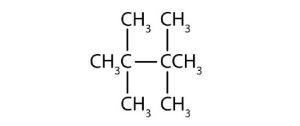 ↵
↵- 2,2,4,4-tetramethylpentane ↵
- Common names are widely used but not very systematic; IUPAC names identify a parent compound and name other groups as substituents. ↵
-
- CH3CH2CH2CH2CH2CH3; hexane
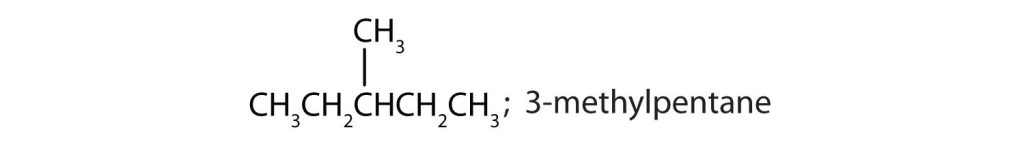
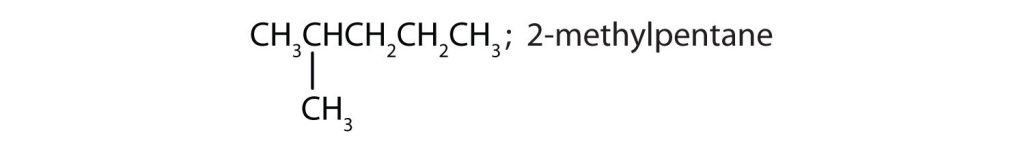
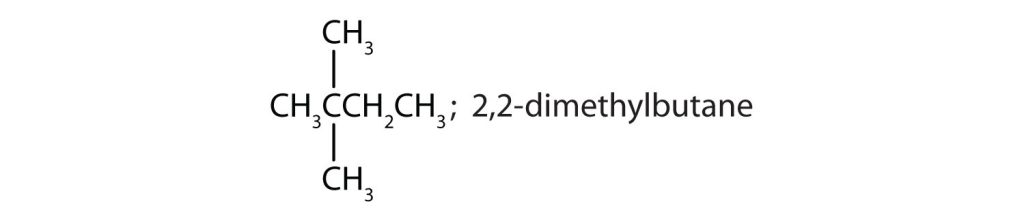
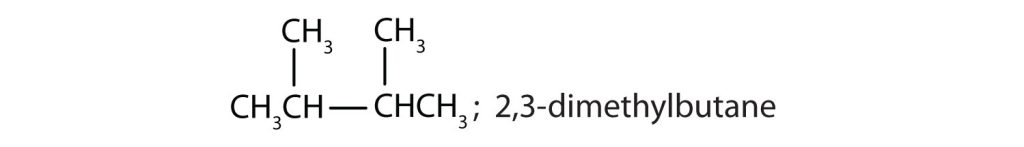 ↵
↵- C8H16 ↵
- ethylcyclopropane ↵
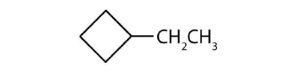 ↵
↵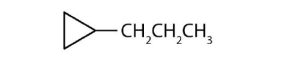 ↵
↵- cyclopentyl bromide ↵
- cyclohexyl chloride ↵
- 1,1,1,2-tetrafluoroethane ↵
- 1,1,1-trifluoro-2,2-dichloroethane ↵
- CH3Cl ↵
- CHCl3 ↵
- CH3CH2CH2Br, propyl bromide, 1-bromopropane; CH3CHBrCH3, isopropyl bromide, 2-bromopropane ↵
- compounds containing Cl, F, and C; by releasing Cl atoms in the stratosphere ↵
- most readily: F2; least readily: I2 ↵
- Alkanes are nonpolar; they do not attract ions. ↵
- C3H8 + 5O2 → 3CO2 + 4H2O ↵
- 2C8H18 + 25O2 → 16CO2 + 18H2O ↵

