Chapter 19 – Summary
19.1 Alkanes, Alkenes, Alkynes and Aromatic Hydrocarbons
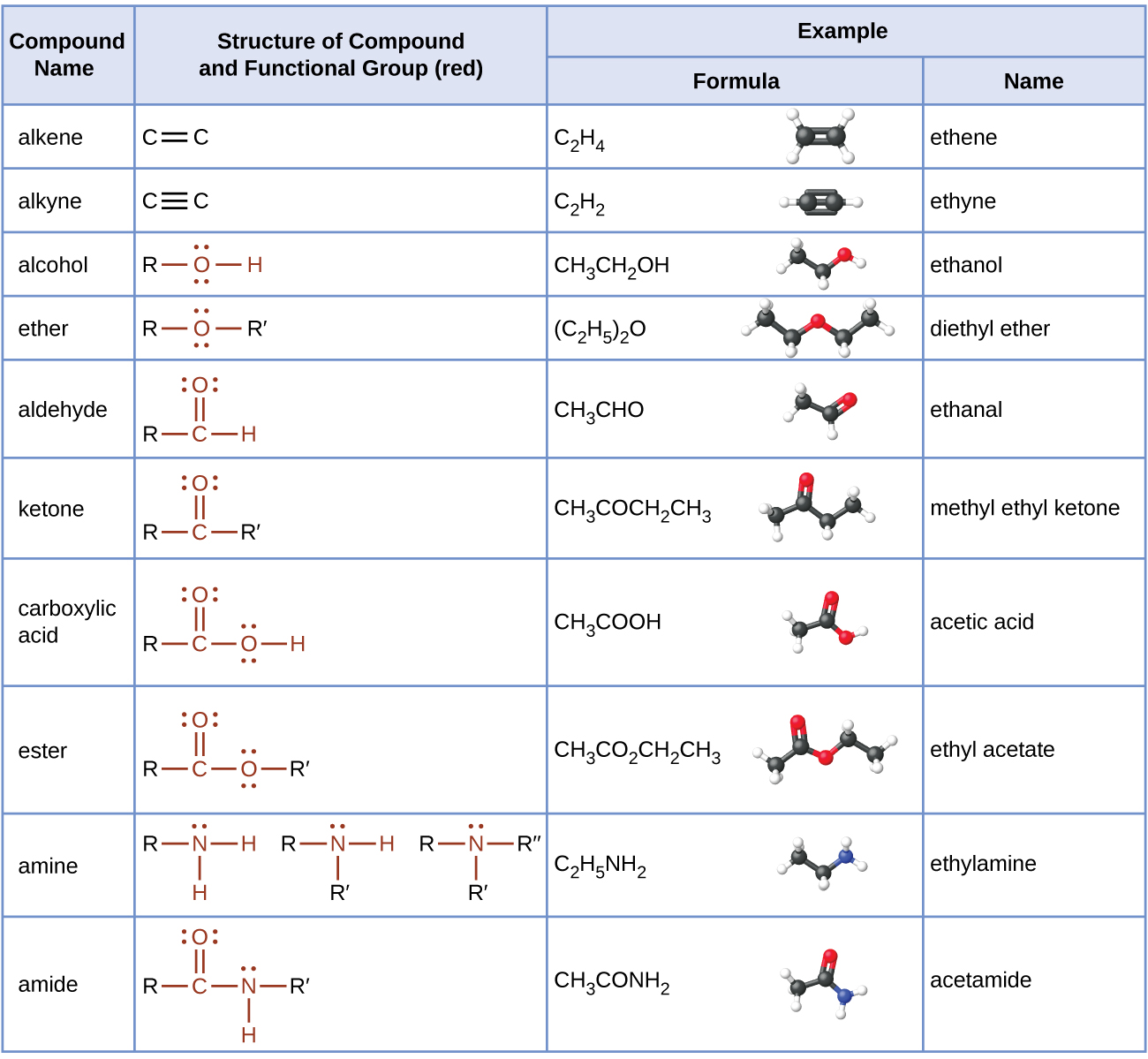
Strong, stable bonds between carbon atoms produce complex molecules containing chains, branches, and rings. The chemistry of these compounds is called organic chemistry. Hydrocarbons are organic compounds composed of only carbon and hydrogen. The alkanes are saturated hydrocarbons—that is, hydrocarbons that contain only single bonds. Alkenes contain one or more carbon-carbon double bonds. Alkynes contain one or more carbon-carbon triple bonds. Aromatic hydrocarbons contain ring structures with delocalized π electron systems.
19.2 Alcohols and Ethers
Many organic compounds that are not hydrocarbons can be thought of as derivatives of hydrocarbons. A hydrocarbon derivative can be formed by replacing one or more hydrogen atoms of a hydrocarbon by a functional group, which contains at least one atom of an element other than carbon or hydrogen. The properties of hydrocarbon derivatives are determined largely by the functional group. The –OH group is the functional group of an alcohol. The –R–O–R– group is the functional group of an ether.
19.3 Aldehydes, Ethers, Carboxylic Acids and Esters
Functional groups related to the carbonyl group include the –CHO group of an aldehyde, the –CO– group of a ketone, the –CO2H group of a carboxylic acid, and the –CO2R group of an ester. The carbonyl group, a carbon-oxygen double bond, is the key structure in these classes of organic molecules: Aldehydes contain at least one hydrogen atom attached to the carbonyl carbon atom, ketones contain two carbon groups attached to the carbonyl carbon atom, carboxylic acids contain a hydroxyl group attached to the carbonyl carbon atom, and esters contain an oxygen atom attached to another carbon group connected to the carbonyl carbon atom. All of these compounds contain oxidized carbon atoms relative to the carbon atom of an alcohol group.
19.4 Amines and Amides
The addition of nitrogen into an organic framework leads to two families of molecules. Compounds containing a nitrogen atom bonded in a hydrocarbon framework are classified as amines. Compounds that have a nitrogen atom bonded to one side of a carbonyl group are classified as amides. Amines are a basic functional group. Amines and carboxylic acids can combine in a condensation reaction to form amides.
19.5 Families of Organic Molecules – Functional Groups
19.6 General Reactions of Carbon
All chemical reactions, whether in the laboratory or in living organisms, follow the same chemical rules. To understand both organic and biological chemistry, it’s necessary to know not just what occurs but also why and how chemical reactions take place. In this chapter, we’ve taken a brief look at the fundamental kinds of organic reactions, we’ve seen why reactions occur, and we’ve seen how reactions can be described.
There are four common kinds of reactions: addition reactions take place when two reactants add together to give a single product; elimination reactions take place when one reactant splits apart to give two products; substitution reactions take place when two reactants exchange parts to give two new products; and rearrangement reactions take place when one reactant undergoes a reorganization of bonds and atoms to give an isomeric product.
Additionally, there are oxidation reactions that occur. Oxidation reactions are those that involve a reaction between hydrocarbons and oxygen producing carbon dioxide and water. An example of an oxidation reaction is combustion.
Lastly, oxidation–reduction reactions in organic chemistry are identified by the change in the number of oxygens in the hydrocarbon skeleton or the number of bonds between carbon and oxygen or carbon and nitrogen.
19.7 Introduction to Green Chemistry
Green chemistry involves inventing new chemicals, new chemical processes and commercial products that reduce chemical hazards and minimize hazardous effects on human health and the environment. The philosophy is based on 12 principles and are summarized by four key ideas: prevent the formation of waste in the first place, employ safer reagents or solvents, implement selective and efficient transformations (reactions), and avoid unnecessary transformations. Current research has many examples of green chemistry in action.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards and Samantha Sullivan Sauer from
- “18.1 Hydrocarbons,” “18.2 Alcohols and Ethers,” “18.3 Aldehydes, Ketones, Carboxylic Acids, and Esters “and “18.4 Amines and Amides” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- 19.6 summary is adapted from: “Ch. 6 Summary” In Organic Chemistry (OpenStax) by John McMurray, licensed under CC BY-NC-SA 4.0 Access for free at Organic Chemistry (OpenStax)
- Oxidation-Reduction reaction section within 19.6 summary is adapted from “23.5: Common Classes of Organic Reactions” In Map: General Chemistry: Principles, Patterns, and Applications (Averill) was authored, remixed, and/or curated by Joshua B. Halpern / LibreTexts / Anonymous, CC BY-NC-SA 3.0

