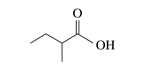
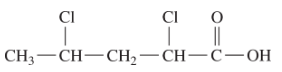25.1 Carboxylic Acids – Structure and Naming
Learning Objectives
By the end of this section, you will be able to:
- Name carboxylic acids with common names.
- Name carboxylic acids according to IUPAC nomenclature.
Structure of Carboxylic Acids
Carboxylic acids occur widely in nature, often combined with alcohols or other functional groups, as in fats, oils, and waxes. They are components of many foods, medicines, and household products (Figure 25.1a.). Carboxylic acids are considered weak acids that can neutralize bases and tend to have a sour or tart taste to them. Not surprisingly, many of them are best known by common names based on Latin and Greek words that describe their source.

A carboxylic acid is an organic compound in which a carbon is double bonded to an oxygen atom (referred to as a carbonyl group) while also being single bonded to a hydroxyl group (-OH). This combination of carbonyl group with a hydroxyl group creates what is known as a carboxyl group, the functional group found in carboxylic acids.
Indigenous Perspectives: Inuit Love Soy Sauce

Soy sauce was first produced in China about 2,200 years ago. It is now commonly used in Inuit culture as the condiment of choice for Arctic Char also known as iKaluk in Inuttut. Spiced soy sauces can contain different carboxylic acids which can add to the flavour profile. A healthy ingredient within soy sauce is Niacin which is an aromatic carboxylic acid (Figure 25.1c.) (Anderson & Rayner-Canham, 2022).
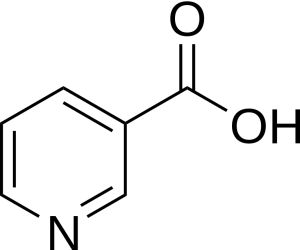
More information about the use of soy sauce in Inuit culture can be found Soy sauce: An essential Inuit condiment in Chem 13 News Magazine.
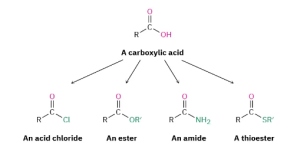
Carboxylic acids, RCO2H, occupy a central place among carbonyl compounds. Not only are they valuable in themselves, they also serve as starting materials for preparing numerous carboxylic acid derivatives such as acid chlorides, esters, amides, and thioesters (Figure 25.1d.). In addition, carboxylic acids are present in the majority of biological pathways.
Common Names of Carboxylic Acids
Many carboxylic acids are named using their common names which use the prefixes: form-, acet-, proprion-, and butyr-. The simplest carboxylic acid, formic acid (HCOOH), was first obtained by the distillation of ants (Latin formica, meaning “ant”) (Figure 25.1e.). The bites of some ants inject formic acid, and the stings of wasps and bees contain formic acid (as well as other poisonous materials).
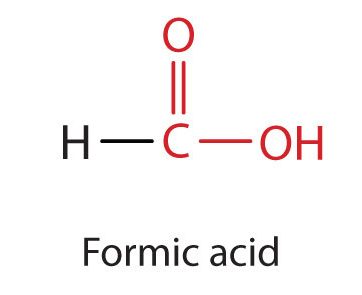
The next higher homolog is acetic acid (Figure 25.1f.), which is made by fermenting cider and honey in the presence of oxygen. This fermentation produces vinegar, a solution containing 4%–10% acetic acid, plus a number of other compounds that add to its flavour. Acetic acid is probably the most familiar weak acid used in educational and industrial chemistry laboratories.
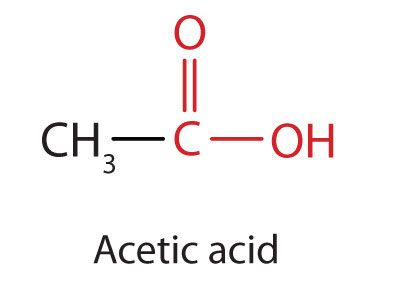
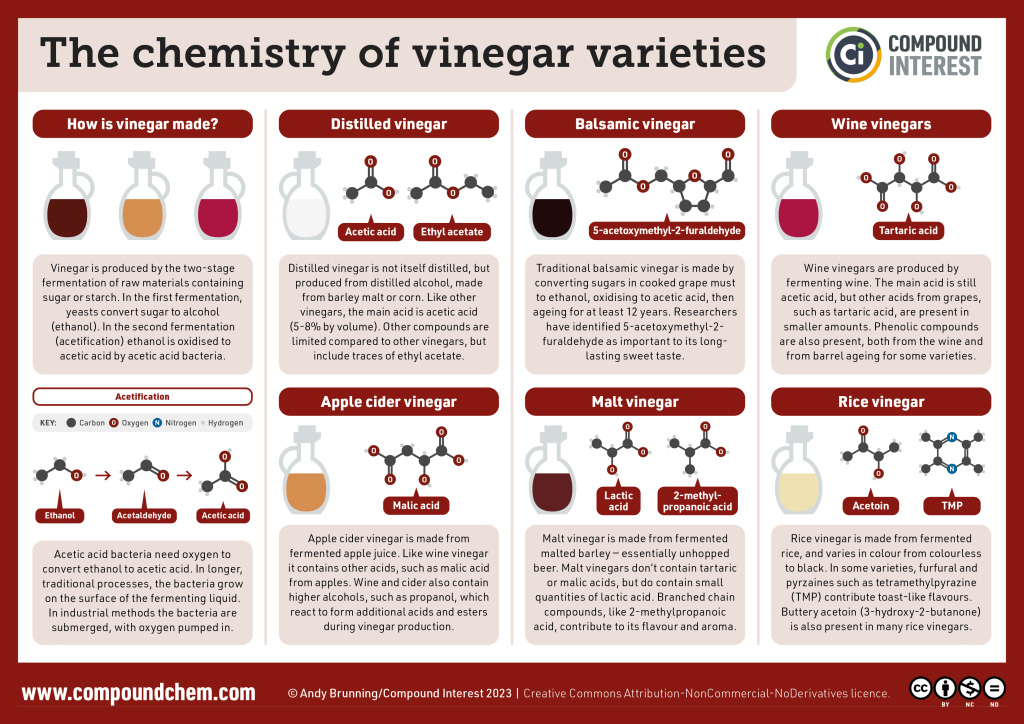
Pure acetic acid solidifies at 16.6°C, only slightly below normal room temperature. In the poorly heated laboratories of the late 19th and early 20th centuries in northern North America and Europe, acetic acid often “froze” on the storage shelf. For that reason, pure acetic acid (sometimes called concentrated acetic acid) came to be known as glacial acetic acid, a name that survives to this day. Vinegar comes in a variety of forms which we commonly use in everyday cooking practices. Infographic 25.1a. showcases the changes to chemical structure that provides us with the differing flavours we experience from some of the vinegar varieties.
Spotlight on Everyday Chemistry: Vinegar
Below we can see the chemical structures that make up the varieties of vinegars we use in everyday cooking practice.
The third homolog, propionic acid (CH3CH2COOH), is seldom encountered in everyday life. The fourth homolog, butyric acid (CH3CH2CH2COOH), is one of the most foul-smelling substances imaginable. It is found in rancid butter and is one of the ingredients of body odour. By recognizing extremely small amounts of this and other chemicals, bloodhounds are able to track fugitives.
Below (Figure 25.1g.) is an example of a carboxylic acid with a substituent group. This acid is named 2-bromo-propanoic acid. 2-bromo-propanoic acid is used in the production of herbicides and the synthesis of pharmaceutical intermediates.
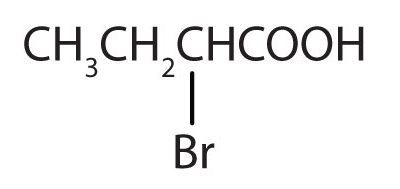
Watch Carboxylic Acids, Typical Acids and Esters – Organic Chemistry – YouTube (4 min)
Video source: Fuse School – Global Education. (2014, August 10). Carboxylic acids, typical acids and esters – Organic Chemistry – Chemistry – Fuse School [Video]. YouTube.
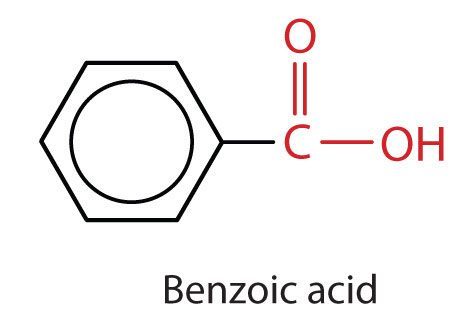
The most simplistic aromatic carboxylic acid in which the carboxyl carbon is attached directly to carbon 1 is called benzoic acid (C6H5COOH) (Figure 25.1h.). When substituent groups are added to benzoic acid, they are numbered in the direction that gives the smallest numbers possible.
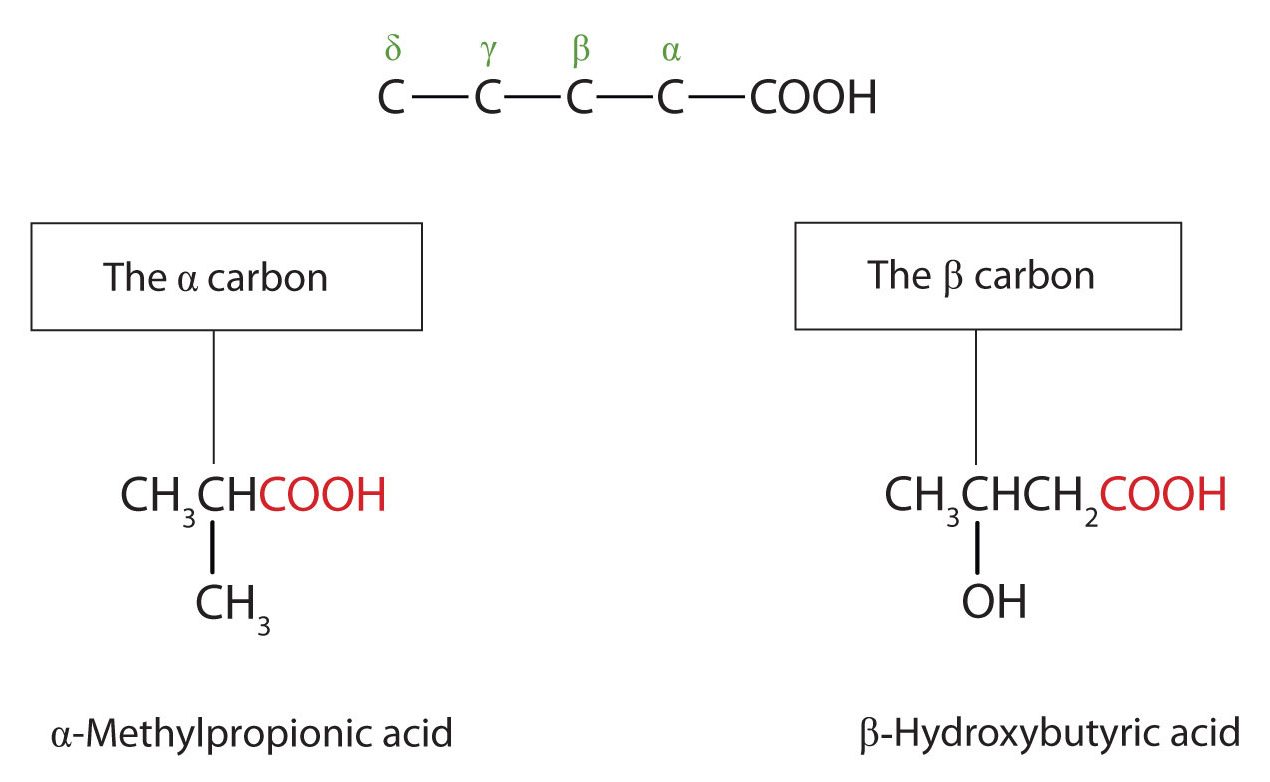
The common names of carboxylic acids use Greek letters (α, β, γ, δ, and so forth), not numbers, to designate the position of substituent groups in acids (Figure 25.1i.). These letters refer to the position of the carbon atom in relation to the carboxyl carbon atom.
IUPAC Naming of Carboxylic Acids
Here are some basic rules for naming carboxylic acids from the International Union of Pure and Applied Chemistry (IUPAC):
- Select the longest carbon chain containing the carboxyl group, this is the parent chain. The -e ending of the parent alkane name is replaced by the suffix -oic acid.
- The carboxyl carbon is always numbered “1” but the number is NOT included in the name.
- Name the substituents attached to the chain in the usual way (providing you with the lowest numbering possible for these groups).
- Aromatic carboxylic acids (ie. with a COOH) directly connected to a benzene ring) are named after the parents compound benzoic acid.
In the IUPAC nomenclature system, the parent hydrocarbon is the one that corresponds to the longest continuous chain (LCC) containing the carboxyl group. The –e ending of the parent alkane is replaced by the suffix –oic and the word acid. For example, the carboxylic acid derived from pentane is pentanoic acid (CH3CH2CH2CH2COOH). As with aldehydes, if there are substituents the carboxyl carbon atom is counted first; numbers are used to indicate the substituted carbon atoms in the parent chain.
Greek letters are used with common names; numbers are used with IUPAC names.
Example 25.1a
Give the common and IUPAC names for each compound.
- ClCH2CH2CH2COOH
-
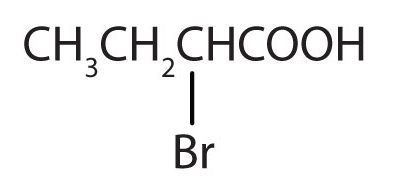
(Credit: Intro Chem: GOB (v. 1.0), CC BY-NC-SA 3.0).
Solution
- The LCC contains four carbon atoms; the compound is therefore named as a substituted butyric (or butanoic) acid.
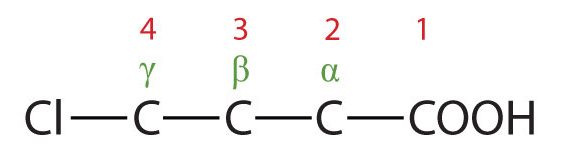
(Credit: Intro Chem: GOB (v. 1.0), CC BY-NC-SA 3.0). The chlorine atom is attached to the γ-carbon in the common system or C4 in the IUPAC system. The compound is γ-chlorobutyric acid or 4-chlorobutanoic acid.
- The LCC contains four carbon atoms; the compound is therefore named as a substituted butyric (or butanoic) acid.
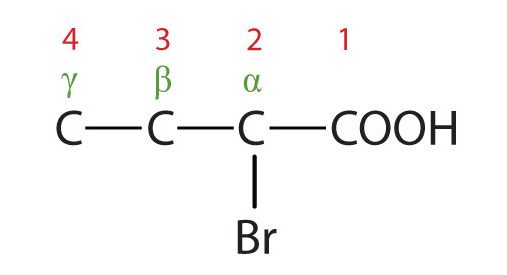
(Credit: Intro Chem: GOB (v. 1.0), CC BY-NC-SA 3.0). The bromine (Br) atom is at the α-carbon in the common system or C2 in the IUPAC system. The compound is α-bromobutyric acid or 2-bromobutanoic acid.
Exercise 25.1a
Write the condensed structural formula for β-chloropropionic acid.
Check Your Answer[1]
Exercise 25.1b
Give the IUPAC name for each compound.
- ClCH2CH2CH2CH2COOH
- (CH3)2CHCH2CHBrCOOH
Check Your Answers:[2]
Exercise 25.1c
Give the IUPAC name for each compound.
Check Your Answer[3]
Image source: Adapted from course materials by Caryn Fahey and JR van Haarlem.
Dicarboxylic Acids
A dicarboxylic acids is an organic compound containing two carboxyl groups (-COOH) often referred to as a diacid. The general molecular formula for dicarboxylic acids can be written as HO−R−COOH. Dicarboxylic acids are used in the preparation of copolymers such as polyamides and polyesters. The most commonly used diacid in industry is adipic acid, which is a precursor to nylon production. Other examples of diacids include aspartic acid and glutamic acid, both of which are amino acids in the human body.
Example 25.1b
Name the following dicarboxylic acids.
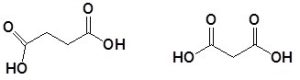
Solution:
- butanedioic acid
- propanedioic acid
Example 25.1b source: Organic Chemistry (Vollhardt & Schore), CC BY-NC-SA 4.0
Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey from:
- “Why This Chapter?” In Organic Chemistry (Open Stax) by John McMurry licensed under CC BY-NC-SA 4.0. Access for free at Organic Chemistry (Open Stax)
- “15.1: Carboxylic Acids – Structures and Names” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- “19.1: Naming the Carboxylic Acids” by Steven Farmer & William Reusch In Map: Organic Chemistry (Vollhardt and Schore), CC BY-NC-SA 4.0
References cited in-text
Anderson, C. C., & Rayner-Canham, G. (2022, Fall). Soy sauce: An essential Inuit condiment. Chem 13 News Magazine.
-
Propionic acid has three carbon atoms: C–C–COOH. Attach a chlorine (Cl) atom to the parent chain at the beta carbon atom, the second one from the carboxyl group: Cl–C–C–COOH. Then add enough hydrogen atoms to give each carbon atom four bonds: ClCH2CH2COOH.
↵
-
a. 5-chloropentanoic acid, b. 1-bromo-5-methylpentanoic acid
↵
-
1) 2-methylbutanoic acid 2) 2,4-dichloropentanoic acid
↵
An organic compound that has a carboxyl functional group.
a combination of two functional groups attached to a single carbon atom. These two functional groups include; a single bonded hydroxyl (OH) group and a double bonded carbonyl (O) group.Carboxyl is often seen as COOH.
An organic compound which contains 2 carboxyl groups. These organic molecules show similar chemical behaviour and reactivity to monocayboxylic acids.