19.4 Amines and Amides
Learning Objectives
- Classify amines and amides
Amines
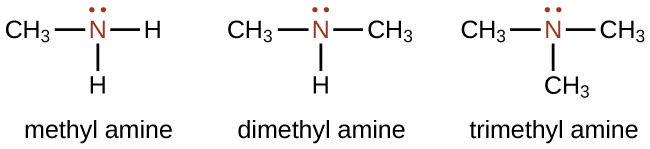
Amines are molecules that contain carbon-nitrogen bonds. The nitrogen atom in an amine has a lone pair of electrons and three bonds to other atoms, either carbon or hydrogen. Various nomenclatures are used to derive names for amines, but all involve the class-identifying suffix –ine as illustrated here for a few simple examples in Figure 19.4a.
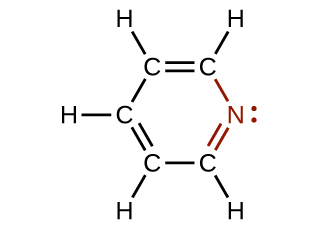
In some amines, the nitrogen atom replaces a carbon atom in an aromatic hydrocarbon. Pyridine (Figure 19.4b) is one such heterocyclic amine. A heterocyclic compound contains atoms of two or more different elements in its ring structure.
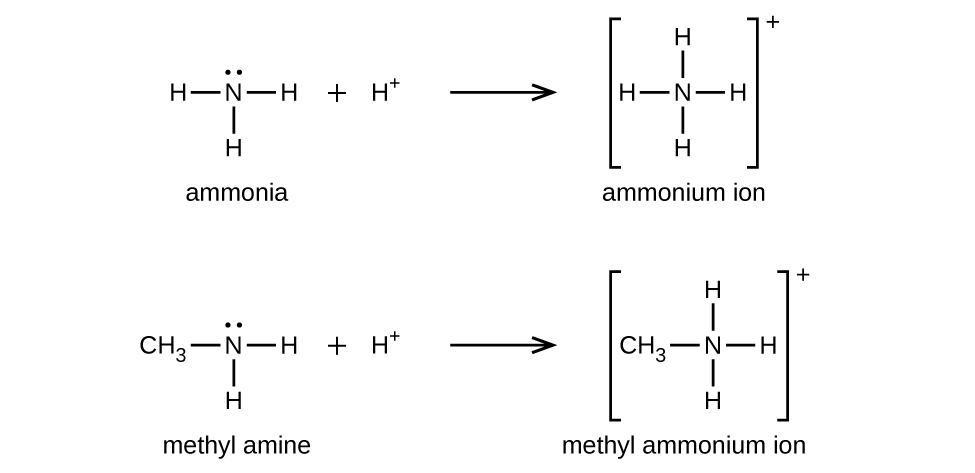
Like ammonia, amines are weak bases (Figure 19.4c), due to the lone pair of electrons on their nitrogen atoms:
The basicity of an amine’s nitrogen atom plays an important role in much of the compound’s chemistry. Amine functional groups are found in a wide variety of compounds, including natural and synthetic dyes, polymers, vitamins, and medications such as penicillin and codeine. They are also found in many molecules essential to life, such as amino acids, hormones, neurotransmitters, and DNA.
Spotlight on Everyday Chemistry: Addictive Alkaloids
Since ancient times, plants have been used for medicinal purposes. One class of substances, called alkaloids, found in many of these plants has been isolated and found to contain cyclic molecules with an amine functional group. These amines are bases. They can react with H3O+ in a dilute acid to form an ammonium salt, and this property is used to extract them from the plant:
The name alkaloid means “like an alkali.” Thus, an alkaloid reacts with acid. The free compound can be recovered after extraction by reaction with a base:
The structures of many naturally occurring alkaloids have profound physiological and psychotropic effects in humans. Examples of these drugs include nicotine, morphine, codeine, and heroin (Figure 19.4d). The plant produces these substances, collectively called secondary plant compounds, as chemical defenses against the numerous pests that attempt to feed on the plant:
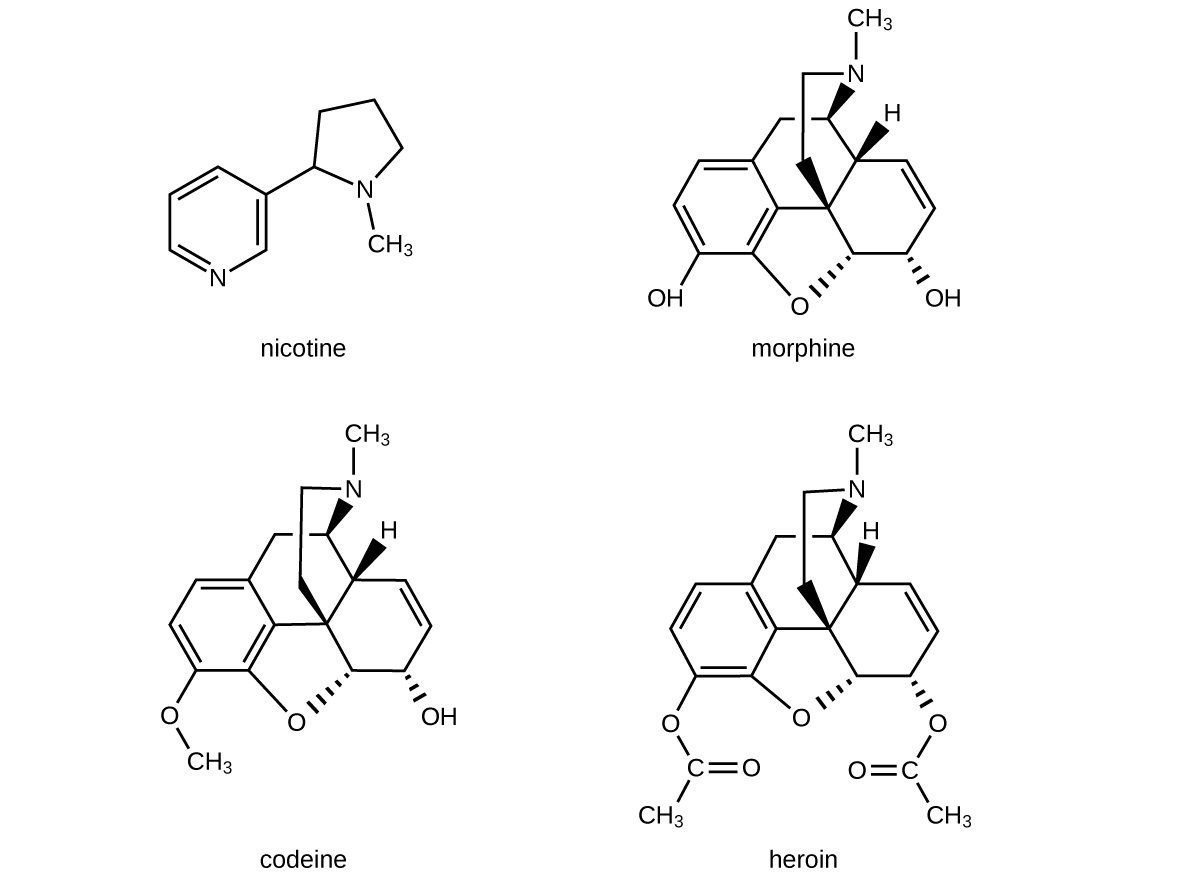
In these diagrams, as is common in representing structures of large organic compounds, carbon atoms in the rings and the hydrogen atoms bonded to them have been omitted for clarity. The solid wedges indicate bonds that extend out of the page. The dashed wedges indicate bonds that extend into the page. Notice that small changes to a part of the molecule change the properties of morphine, codeine, and heroin. Morphine, a strong narcotic used to relieve pain, contains two hydroxyl functional groups, located at the bottom of the molecule in this structural formula. Changing one of these hydroxyl groups to a methyl ether group forms codeine, a less potent drug used as a local anesthetic. If both hydroxyl groups are converted to esters of acetic acid, the powerfully addictive drug heroin results (Figure 19.4e).

Read more about the colours of poppies in Compound Interest: The chemistry of poppies: colours and opium (compoundchem.com).
Furthermore, in some foods that contain poppy seeds, trace amounts of codeine and morphine are present up to 48 hours in urine. In Hungary, there is a sweet roll filled with poppy seeds along with a walnut or chestnut paste, known as Bejgli often served at Christmas. For more information on the Bejgli dessert, see Compound Chemistry Advent 2023.
Amides
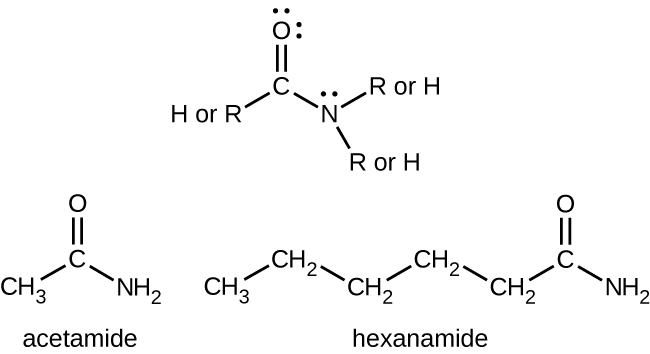
Amides are molecules that contain nitrogen atoms connected to the carbon atom of a carbonyl group (Figure 19.4f). Like amines, various nomenclature rules may be used to name amides, but all include use of the class-specific suffix -amide:
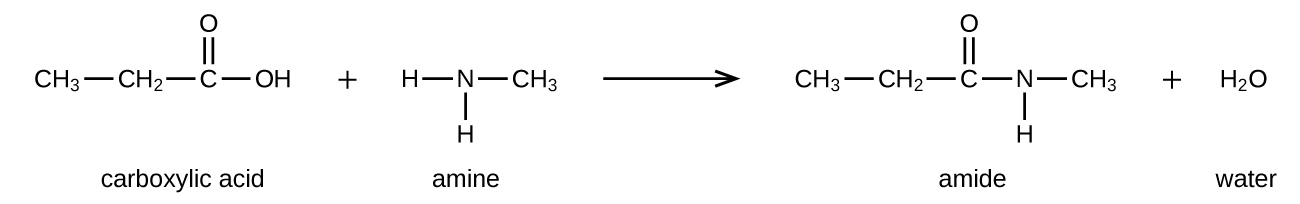
Amides can be produced when carboxylic acids react with amines or ammonia in a process called amidation (Figure 19.4g). A water molecule is eliminated from the reaction, and the amide is formed from the remaining pieces of the carboxylic acid and the amine.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from “18.4 Amines and Amides” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
organic molecule in which a nitrogen atom is bonded to one or more alkyl group
organic molecule that features a nitrogen atom connected to the carbon atom in a carbonyl group

