26.2 Amines – Physical Properties
Learning Objectives
By the end of this section, you will be able to:
- Explain why the boiling points of primary and secondary amines are higher than those of alkanes or ethers of similar molar mass but are lower than those of alcohols.
- Compare the boiling points of tertiary amines with alcohols, alkanes, and ethers of similar molar mass.
- Compare the solubilities in water of amines of five or fewer carbon atoms with the solubilities of comparable alkanes and alcohols in waterProperties of Amines
Properties of Amines
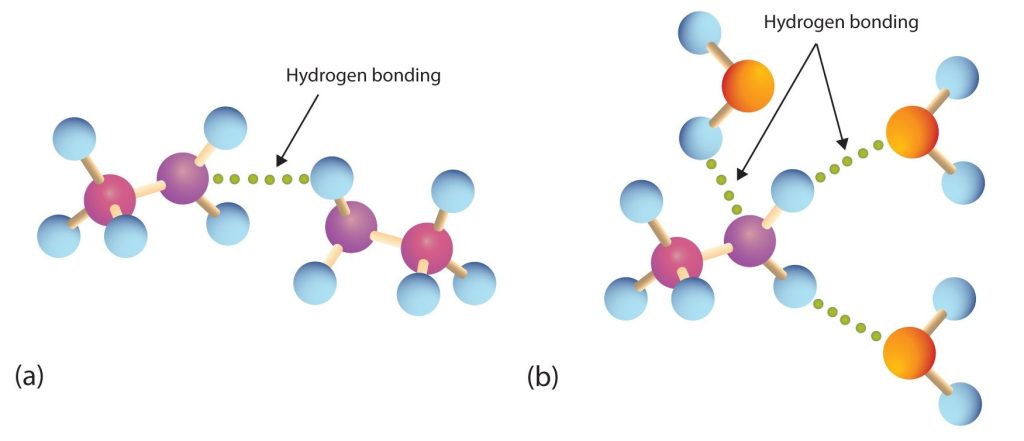
Primary and secondary amines have hydrogen atoms bonded to an nitrogen atom and are therefore capable of hydrogen bonding (part (a) of Figure 26.2a.), although not as strongly as alcohol molecules (which have hydrogen atoms bonded to an oxygen atom, which is more electronegative than nitrogen). These amines boil at higher temperatures than alkanes but at lower temperatures than alcohols of comparable molar mass. For example, compare the boiling point of methylamine (CH3NH2; −6°C) with those of ethane (CH3CH3; −89°C) and methanol (CH3OH; 65°C). Tertiary amines have no hydrogen atom bonded to the nitrogen atom and so cannot participate in intermolecular hydrogen bonding. They have boiling points comparable to those of ethers (Table 26.2a.).
| Name | Condensed Structural Formula | Class | Molar Mass | Boiling Point (°C) | Solubility at 25°C (g/100 g Water) |
|---|---|---|---|---|---|
| butylamine | CH3CH2CH2CH2NH2 | 1° | 73 | 78 | miscible |
| diethylamine | (CH3CH2)2NH | 2° | 73 | 55 | miscible |
| butyl alcohol | CH3CH2CH2CH2OH | — | 74 | 118 | 8 |
| dipropylamine | (CH3CH2CH2)2NH | 2° | 101 | 111 | 4 |
| triethylamine | (CH3CH2)3N | 3° | 101 | 90 | 14 |
| dipropyl ether | (CH3CH2CH2)2O | — | 102 | 91 | 0.25 |
Source: “15.11: Physical Properties of Amines” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
All three classes of amines can engage in hydrogen bonding with water (Figure 26.2a.). Amines of low molar mass are quite soluble in water; the borderline of solubility in water is at five or six carbon atoms (Table 26.2a.).
Spotlight on Everyday Chemistry: Hazards with Amines
Amines have “interesting” odours. The simple ones smell very much like ammonia. Higher aliphatic amines smell like decaying fish. Or perhaps we should put it the other way around: Decaying fish give off odorous amines. The stench of rotting fish is due in part to two diamines: putrescine and cadaverine. They arise from the decarboxylation of ornithine and lysine, respectively, amino acids that are found in animal cells.
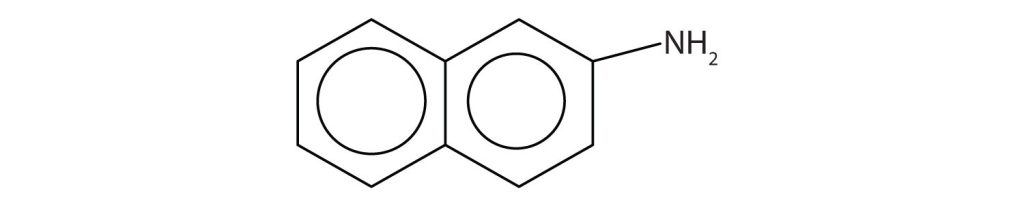
Aromatic amines generally are quite toxic. They are readily absorbed through the skin, and workers must exercise caution when handling these compounds. Several aromatic amines, including β-naphthylamine (Figure 26.2b.), are potent carcinogens.
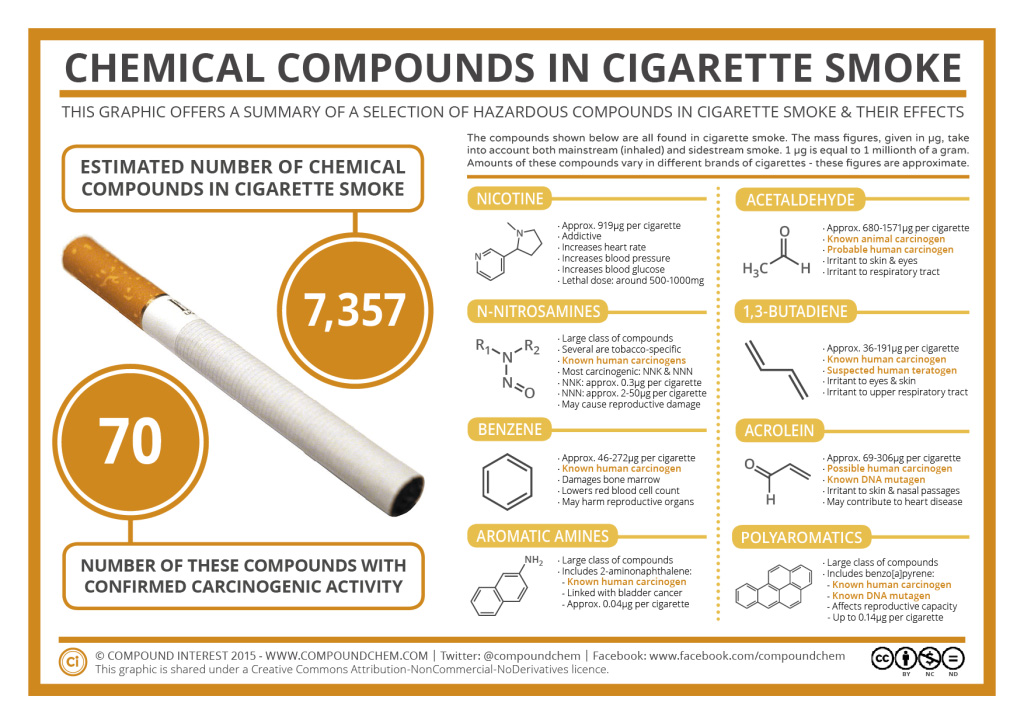
Cigarettes and cigarette smoke also have amine-based compounds. Read more in Infographic 26.2a.
Exercise 26.2a
-
Which compound has the higher boiling point, CH3CH2CH2CH2CH2NH2 or CH3CH2CH2CH2CH2CH3? Explain.
-
Which compound is more soluble in water, CH3CH2CH2CH2CH3 or CH3CH2NHCH2CH3? Explain.
Check Your Answers:[1]
Spotlight on Everyday Chemistry: Scientist Dr. Nadine Borduas
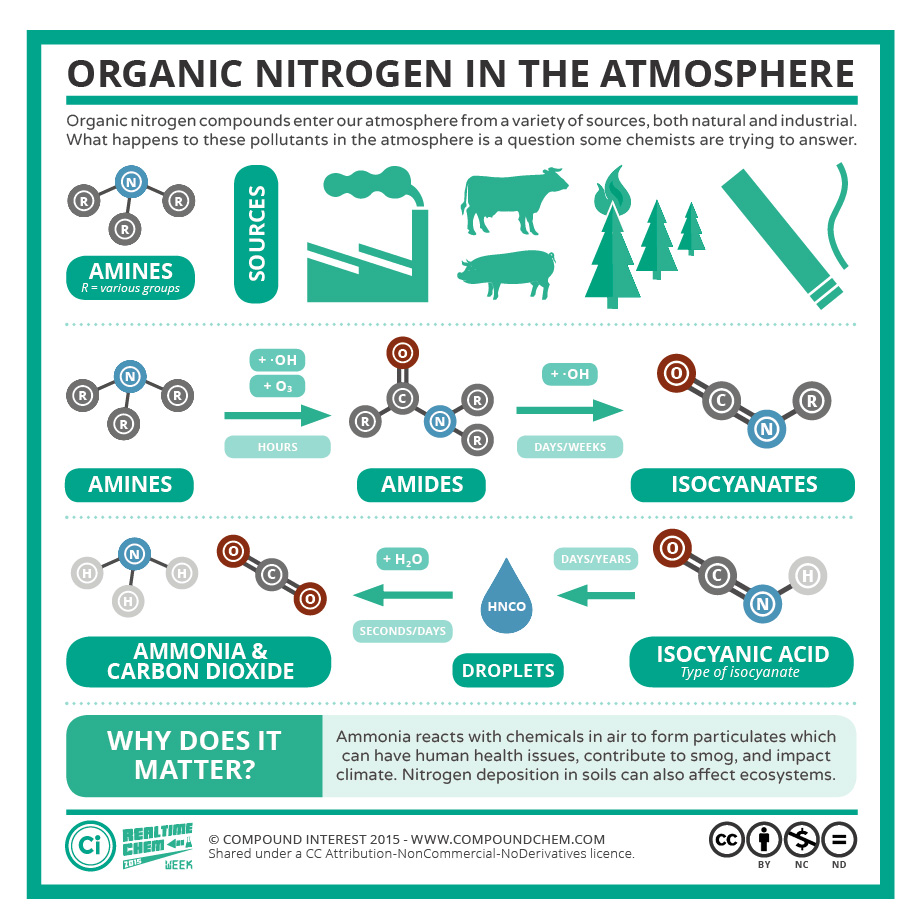
Research on nitrogen-containing amine compounds in the atmosphere is explained in Infographic 26.2b.

Attribution & References
Except where otherwise noted, this page is adapted by Caryn Fahey and Samantha Sullivan Sauer from
- “16.3: Properties of Amines” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.), CC BY-NC-SA 3.0, a remixed version of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 which is a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC-SA 3.0
-
- CH3CH2CH2CH2CH2NH2 because the nitrogen-to-hydrogen (N–H) bonds can engage in hydrogen bonding; CH3CH2CH2CH2CH2CH3 cannot engage in hydrogen bonding
- CH3CH2NHCH2CH3 because amines can engage in hydrogen bonding with water; alkanes cannot engage in hydrogen bonding ↵

