22.1 Alkenes and Alkynes – Structure and Naming
Learning Objectives
- Identify the difference between saturated and unsaturated hydrocarbons.
- Describe the functional groups, alkenes and alkynes.
- Properly name alkene and alkynes using the IUPAC naming system
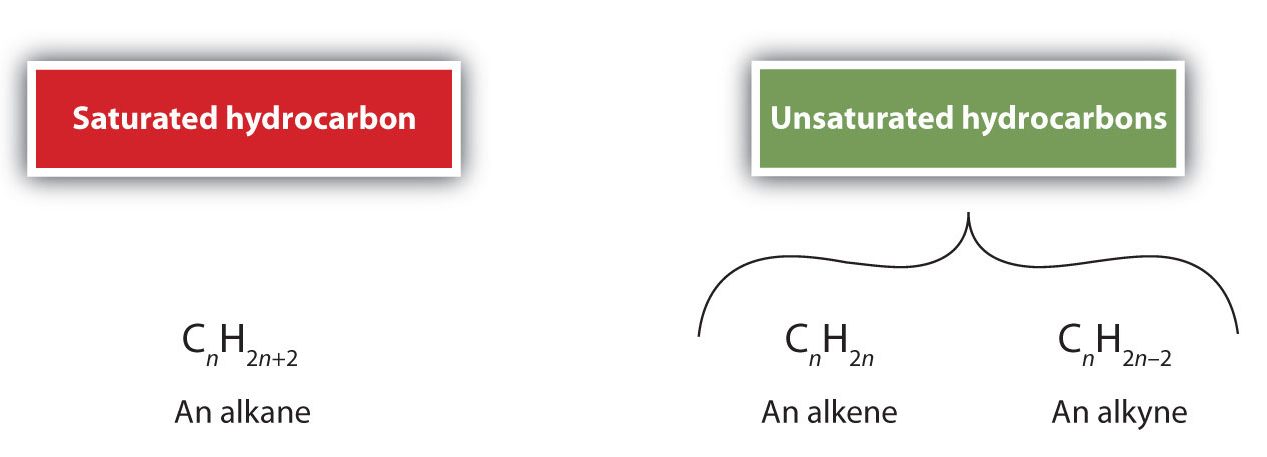
As noted before, alkenes are hydrocarbons with carbon-to-carbon double bonds (R2C=CR2) and alkynes are hydrocarbons with carbon-to-carbon triple bonds (R–C≡C–R). Collectively, they are called unsaturated hydrocarbons because they have fewer hydrogen atoms than does an alkane with the same number of carbon atoms, as is indicated in the following general formulas in Figure 22.1a.
You have likely heard of unsaturated fats. These are complex organic molecules with long chains of carbon atoms, which contain at least one double bond between carbon atoms. Double and triple bonds give rise to a different geometry around the carbon atom that participates in them, leading to important differences in molecular shape and properties. The differing geometries are responsible for the different properties of unsaturated versus saturated fats.
Alkenes
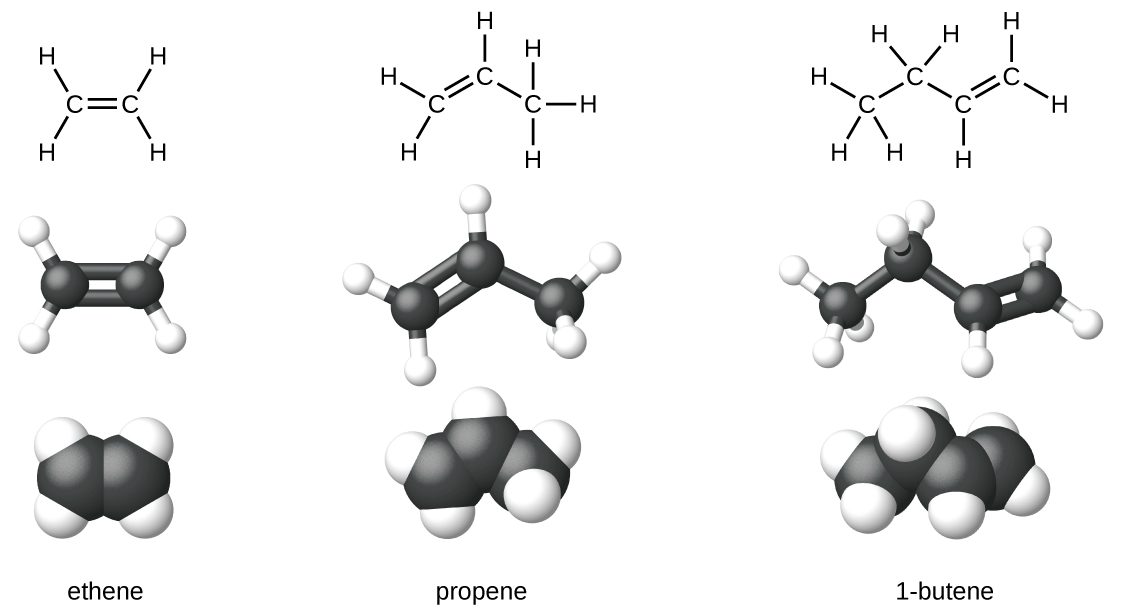
Ethene, C2H4, is the simplest alkene. Each carbon atom in ethene, commonly called ethylene, has a trigonal planar structure. The second member of the series is propene (propylene) (Figure 22.1b.); the butene isomers follow in the series. Four carbon atoms in the chain of butene allows for the formation of isomers based on the position of the double bond, as well as a new form of isomerism.
Some representative alkenes—their names, structures, and physical properties—are given in Table 22.1a. In general, as the chain length increases, the melting and boiling points increase. In comparison to alkanes, alkenes have higher melting points but lower boiling points. For instance, looking at propane versus propene, propane has a melting point of -190oC and a boiling point of -42oC, whereas propene has a melting point of -185oC and a boiling point of -47oC. When comparing alkenes to alkanes, refer to Table 20.1b Properties of Some Alkanes.
| IUPAC Name | Molecular Formula | Condensed Structural Formula | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|---|---|
| ethene | C2H4 | CH2=CH2 | –169 | –104 |
| propene | C3H6 | CH2=CHCH3 | –185 | –47 |
| 1-butene | C4H8 | CH2=CHCH2CH3 | –185 | –6 |
| 1-pentene | C5H10 | CH2=CH(CH2)2CH3 | –138 | 30 |
| 1-hexene | C6H12 | CH2=CH(CH2)3CH3 | –140 | 63 |
| 1-heptene | C7H14 | CH2=CH(CH2)4CH3 | –119 | 94 |
| 1-octene | C8H16 | CH2=CH(CH2)5CH3 | –102 | 121 |
Table source: “13.1: Alkenes: Structures and Names” In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0.
We used only condensed structural formulas in Table 1. Thus, CH2=CH2 is illustrated in Figure 22.1c.
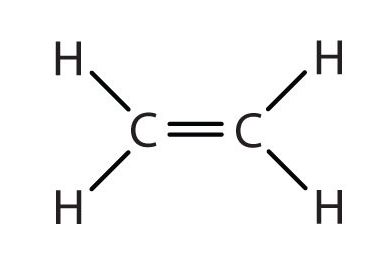
The double bond is shared by the two carbons and does not involve the hydrogen atoms, although the condensed formula does not make this point obvious. Note that the molecular formula for ethene is C2H4, whereas that for ethane is C2H6.
The first two alkenes in Table 22.1a., ethene and propene, are most often called by their common names—ethylene and propylene, respectively (Figure 22.1d.). Ethylene is a major commercial chemical. The US chemical industry produces about 25 billion kilograms of ethylene annually, more than any other synthetic organic chemical. More than half of this ethylene goes into the manufacture of polyethylene, one of the most familiar plastics. Propylene is also an important industrial chemical. It is converted to plastics, isopropyl alcohol, and a variety of other products.
Naming Alkenes
1. Name the parent hydrocarbon. Find the longest carbon chain containing the double bond, and name the compound accordingly, using the suffix -ene as in Figure 22.1e.
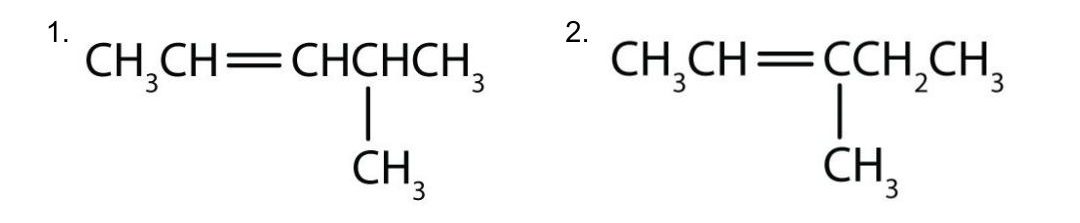
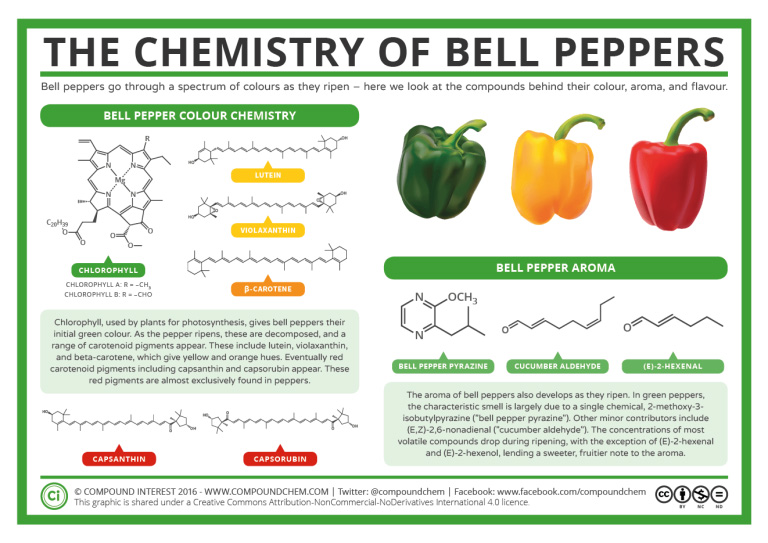
2. Number the carbon atoms in the chain. Begin at the end nearer the double bond or, if the double bond is equidistant from the two ends, begin at the end nearer the first branch point. This rule ensures that the double-bond carbons receive the lowest possible numbers such as those examples shown in Figure 22.1f.
3. Write the full name. Number the substituents according to their positions in the chain, and list them alphabetically. Indicate the position of the double bond by giving the number of the first alkene carbon and placing that number directly before the parent name. If more than one double bond is present, indicate the position of each and use one of the suffixes -diene, -triene, and so on.
Solution
- The longest chain containing the double bond has five carbon atoms, so the compound is a pentene (rule 1). To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 2-pentene. There is a methyl group on the fourth carbon atom (rule 3), so the compound’s name is 4-methyl-2-pentene.
- The longest chain containing the double bond has five carbon atoms, so the parent compound is a pentene (rule 1). To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 2-pentene. There is a methyl group on the third carbon atom (rule 3), so the compound’s name is 3-methyl-2-pentene.
Example 22.1b
Name this compound.
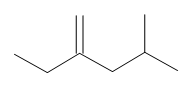
Solution:
First, find the longest chain that contains the C=C bond. Notice that is it not the six-carbon chain across the page. The longest chain is 5 carbons. The C=C bond gets the lowest possible number. This compound is 2-ethyl-4-methylpent-1-ene.
Activity source: Exercise 22.1b is created by Samantha Sullivan Sauer, using images from Biovia Draw, licensed under CC BY-NC 4.0
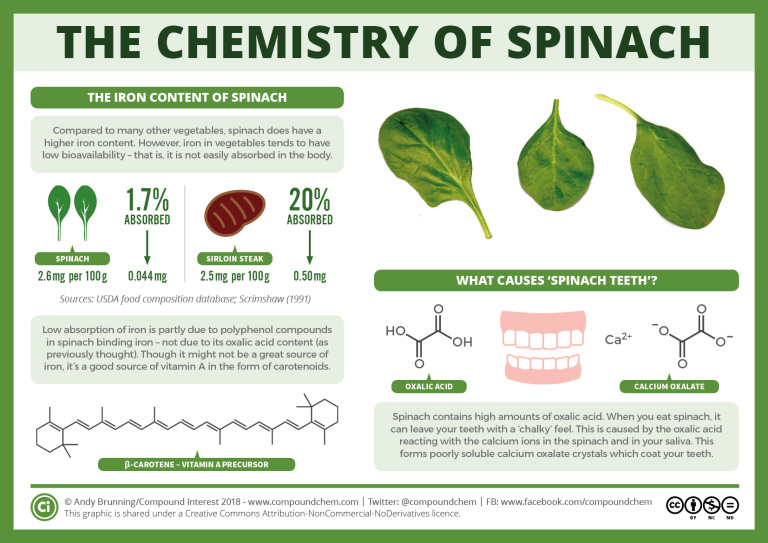
Beta-carotene (found in spinach), capsanthin and capsorubin (both found in peppers), consist of long chained alkenes. Check out the infographics 22.1a. and 22.1b. for more details.
Spotlight on Everyday Chemistry: Spinach and Bell Peppers
The vitamin A precursor found in both spinach and bell peppers is beta-carotene, an alkene. Beta-carotene consists of a long carbon chain with multiple double bonds with a substituted cyclohexene at each end. In addition, pigments and scents within bell peppers are alkenes. Specifically, chlorophyll contributes to the green pigment found in bell peppers and many other green plants. For more information see infographic 22.1a. and 22.1b.
Naming Cycloalkenes
Cycloalkenes are named similarly, but because there is no chain end to begin from, we number the cycloalkene so that the double bond is between C1 and C2 and the first substituent has as low a number as possible. It’s not necessary to indicate the position of the double bond in the name because it’s always between C1 and C2. As with open-chain alkenes, the newer but not yet widely accepted naming rules place the locant immediately before the suffix in a cyclic alkene.
Draw the structure for each compound.
- 3-methyl-2-pentene
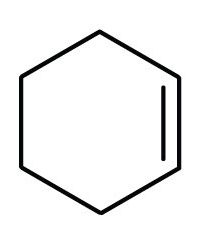
- cyclohexene
Solution
- First write the parent chain of five carbon atoms: C–C–C–C–C. Then add the double bond between the second and third carbon atoms:
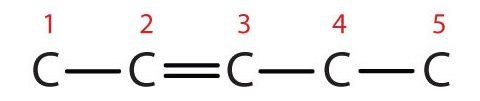
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
Now place the methyl group on the third carbon atom and add enough hydrogen atoms to give each carbon atom a total of four bonds.
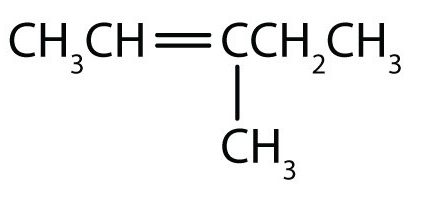
- First, consider what each of the three parts of the name means. Cyclo means a ring compound, hex means 6 carbon atoms, and –ene means a double bond.
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
Exercise 22.1a
Name each compound.
-
CH3CH2CH2CH2CH2CH=CHCH3
-
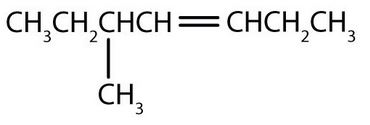
Check Your Answers:[1]
Exercise source: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0
Spotlight on Everyday Chemistry: Ethiopian Rooster Doro Wat
Doro wat is a common dish served at Christmas in Ethiopia. It consists of a spicy chicken stew with the flavours arising from alkenes and cycloalkenes. Three spices in particular are sotolon (flavour in fenugreek), linalool (flavour in coriander seeds) and 1,8-cineole (flavour in cardamon seeds). Linalool is shown below in Figure 22.1i.
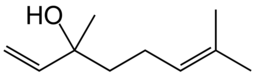
For more information on this dish refer to the infographic from Compound Interest: The Chemistry Advent Calendar 2023 (compoundchem.com).
Alkynes
Hydrocarbon molecules with one or more triple bonds are called alkynes; they make up another series of unsaturated hydrocarbons. Two carbon atoms joined by a triple bond have bond angles of 180°, giving these types of bonds a linear shape.
The simplest member of the alkyne series is ethyne, C2H2, commonly called acetylene as shown in Figure 22.1j. The Lewis structure for ethyne, a linear molecule, is:
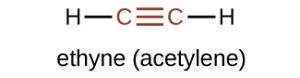
Acetylene is used in oxyacetylene torches for cutting and welding metals. The flame from such a torch can be very hot. Most acetylene, however, is converted to chemical intermediates that are used to make vinyl and acrylic plastics, fibers, resins, and a variety of other products.
Alkynes are similar to alkenes in both physical and chemical properties. For example, alkynes undergo many of the typical addition reactions of alkenes. Again, when comparing alkynes to alkanes, refer to Table 20.1b. Properties of Some Alkanes.
Naming Alkynes
The International Union of Pure and Applied Chemistry (IUPAC) names for alkynes parallel those of alkenes, except that the family ending is –yne rather than –ene.
Alkynes follow the same naming rules as alkenes, using the same stem as alkanes, however they end in -yne to identify it as an alkyne.
Alkyne nomenclature follows the general rules for hydrocarbons replacing with the suffix -yne, and the position of the triple bond is indicated by giving the number of the first alkyne carbon in the chain.
- Numbering the main chain begins at the end nearer the triple bond so that the triple bond receives as low a number as possible as illustrated in Figure 22.1k.
Compounds with more than one triple bond are called diynes, triynes, and so forth; compounds containing both double and triple bonds are called enynes (not ynenes).
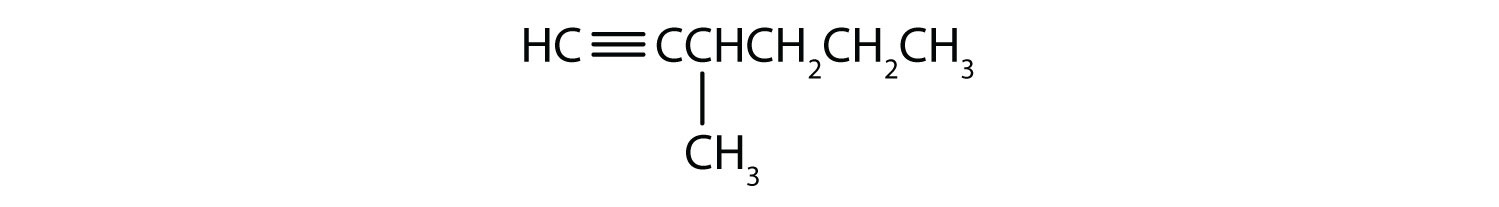
- Numbering of an enyne chain starts from the end nearer the first multiple bond, whether double or triple. When there is a choice in numbering, double bonds receive lower numbers than triple bonds as shown in Figure 22.1l.
For a summary on how to name alkyne, watch Naming Alkynes shown below.
Watch Naming Alkynes – IUPAC Nomenclature & Common Names – Youtube (13 min).
Video Source: The Organic Chemistry Tutor. (2018, April 28). Naming Alkynes – IUPAC Nomenclature & Common Names [Video]. YouTube.
Exercise 22.1b
Draw the structure for each compound.
- acetylene
- 3-methyl-1-hexyne
Check Your Answers:[2]
Exercise source: Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
Exercise 22.1c
Name each alkyne.
- CH3CH2CH2C≡CH
- CH3CH2CH2C≡CCH3
Check Your Answers:[3]
Exercise source: Intro Chem: GOB (v. 1.0), CC BY-NC 3.0
Attribution & References
Except where otherwise noted, this page is adapted by David Wegman from
- “13.1: Alkenes and Alkynes” and “13.2: Naming Alkenes and Alkynes” In Map: Fundamentals of General Organic and Biological Chemistry (McMurry et al.) , CC BY-NC-SA 3.0. / A derivative of Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0. Attributions from original source:
- “20.1: Hydrocarbons” In Chemistry 1e (Open Stax) a LibreTexts version of Chemistry 2e (OpenStax), CC BY 4.0.
- “13.6: Alkynes” In Basics of GOB (Ball et al.), CC BY-NC-SA 4.0 a LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
- Content under heading “Alkenes” is also mixed with “13.1: Alkenes- Structures and Names” In Basics of GOB (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott, CC BY-NC-SA 4.0 / A LibreTexts version of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0
- “7.3 Naming Alkenes“, “9.1 Naming Alkynes” In Organic Chemistry (OpenStax) by John McMurray, CC BY-NC-SA 4.0. Access for free at Organic Chemistry (OpenStax)
molecule consisting of carbon and hydrogen containing at least one carbon-carbon double bond
molecule consisting of carbon and hydrogen containing at least one carbon-carbon triple bond