19.1 Alkanes, Alkenes, Alkynes and Aromatic Hydrocarbons
Learning Objectives
By the end of this section, you will be able to:
- Classify saturated and unsaturated hydrocarbons, and molecules derived from them
- Identify alkanes, alkenes, alkynes and aromatic hydrocarbons
Alkanes
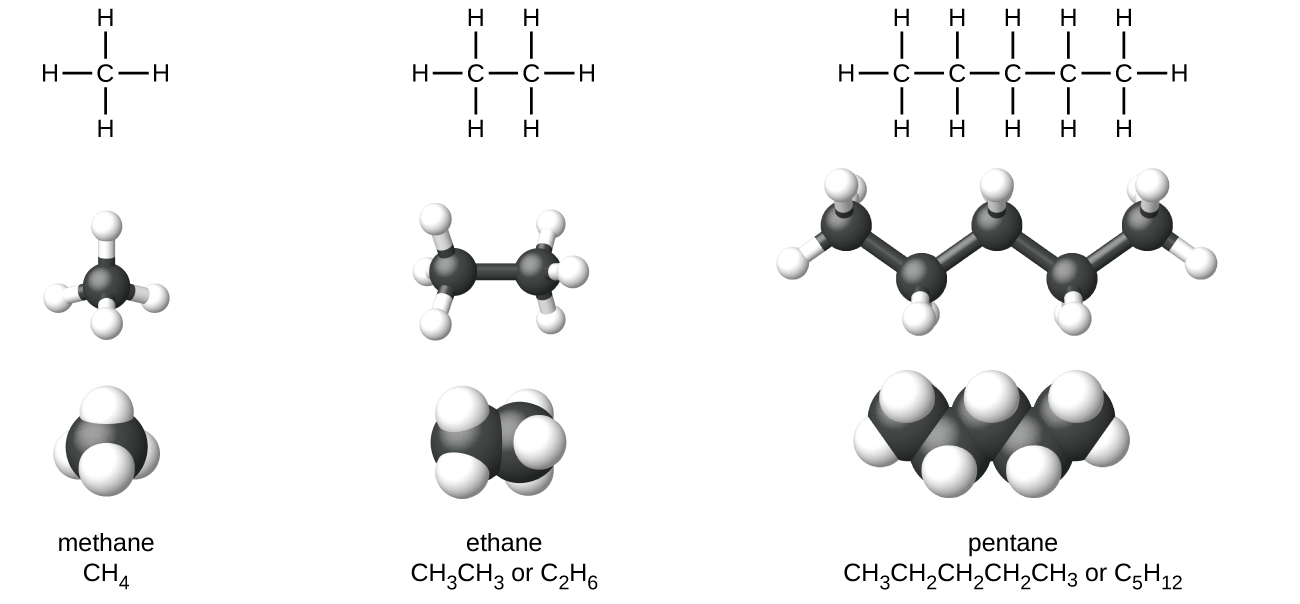
Alkanes, or saturated hydrocarbons, contain only single covalent bonds between carbon atoms. Each of the carbon atoms in an alkane has sp3 hybrid orbitals and is bonded to four other atoms, each of which is either carbon or hydrogen. The Lewis structures and models of methane, ethane, and pentane are illustrated in Figure 19.1a. Carbon chains are usually drawn as straight lines in Lewis structures, but one has to remember that Lewis structures are not intended to indicate the geometry of molecules. Notice that the carbon atoms in the structural models (the ball-and-stick and space-filling models) of the pentane molecule do not lie in a straight line. Because of the sp3 hybridization, the bond angles in carbon chains are close to 109.5°, giving such chains in an alkane a zigzag shape.
The structures of alkanes and other organic molecules may also be represented in a less detailed manner by condensed structural formulas (or simply, condensed formulas). Instead of the usual format for chemical formulas in which each element symbol appears just once, a condensed formula is written to suggest the bonding in the molecule. These formulas have the appearance of a molecular structure from which most or all of the bond symbols have been removed. Condensed structural formulas for ethane and pentane are shown at the bottom of Figure 19.1a.
Alkenes
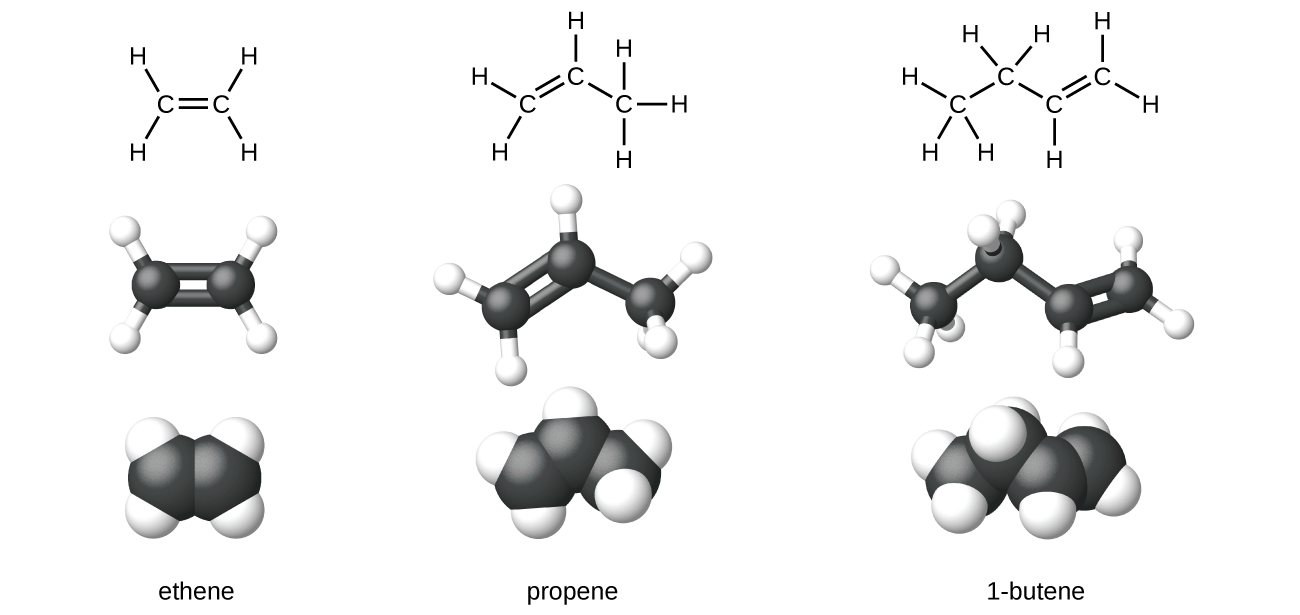
Organic compounds that contain one or more double or triple bonds between carbon atoms are described as unsaturated. You have likely heard of unsaturated fats. These are complex organic molecules with long chains of carbon atoms, which contain at least one double bond between carbon atoms. Unsaturated hydrocarbon molecules that contain one or more double bonds are called alkenes. Carbon atoms linked by a double bond are bound together by two bonds, one σ bond and one π bond. Double and triple bonds give rise to a different geometry around the carbon atom that participates in them, leading to important differences in molecular shape and properties. The differing geometries are responsible for the different properties of unsaturated versus saturated fats.
Ethene, C2H4, is the simplest alkene. Each carbon atom in ethene, commonly called ethylene, has a trigonal planar structure. The second member of the series is propene (propylene) (Figure 19.1b); the butene structures follow in the series.
Ethylene (the common industrial name for ethene) is a basic raw material in the production of polyethylene and other important compounds. Over 135 million tons of ethylene were produced worldwide in 2010 for use in the polymer, petrochemical, and plastic industries. Ethylene is produced industrially in a process called cracking, in which the long hydrocarbon chains in a petroleum mixture are broken into smaller molecules.
Alkynes
Hydrocarbon molecules with one or more triple bonds are called alkynes; they make up another series of unsaturated hydrocarbons. Two carbon atoms joined by a triple bond are bound together by one σ bond and two π bonds. The sp-hybridized carbons involved in the triple bond have bond angles of 180°, giving these types of bonds a linear, rod-like shape. The molecular structure for ethyne, a linear molecule, is:
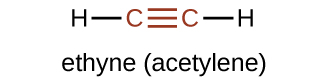
The simplest member of the alkyne series is ethyne, C2H2, commonly called acetylene is represented in Figure 19.1c.
Aromatic Hydrocarbons
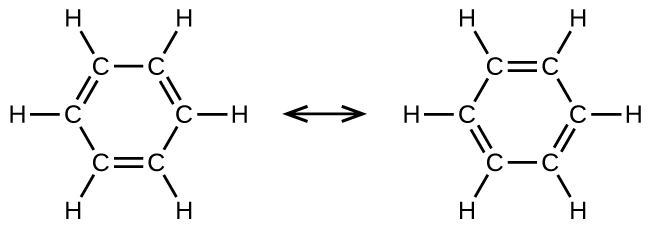
Benzene, C6H6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene is represented in Figure 19.1d. These compounds contain ring structures and exhibit bonding that must be described using the resonance hybrid concept of valence bond theory or the delocalization concept of molecular orbital theory. (To review these concepts, refer to the earlier chapters on chemical bonding). The resonance structures for benzene, C6H6, are:
Valence bond theory describes the benzene molecule and other planar aromatic hydrocarbon molecules as hexagonal rings of sp2-hybridized carbon atoms with the unhybridized p orbital of each carbon atom perpendicular to the plane of the ring. Three valence electrons in the sp2 hybrid orbitals of each carbon atom and the valence electron of each hydrogen atom form the framework of σ bonds in the benzene molecule. The fourth valence electron of each carbon atom is shared with an adjacent carbon atom in their unhybridized p orbitals to yield the π bonds. Benzene does not, however, exhibit the characteristics typical of an alkene. Each of the six bonds between its carbon atoms is equivalent and exhibits properties that are intermediate between those of a C–C single bond and a [latex]\text{C}\;=\;\text{C}[/latex] double bond. To represent this unique bonding, structural formulas for benzene and its derivatives are typically drawn with single bonds between the carbon atoms and a circle within the ring as shown in Figure 19.1e.
There are many derivatives of benzene. The hydrogen atoms can be replaced by many different substituents. Aromatic compounds more readily undergo substitution reactions than addition reactions; replacement of one of the hydrogen atoms with another substituent will leave the delocalized double bonds intact.
Toluene and xylene are important solvents and raw materials in the chemical industry. Styrene is used to produce the polymer polystyrene. These molecules are shown in Figure 19.1f.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from "18.1 Hydrocarbons" In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
molecule consisting of only carbon and hydrogen atoms connected by single (σ) bonds
molecule containing carbon and hydrogen that has only single bonds between carbon atoms
natural or synthetic compound that contains carbon
molecule consisting of carbon and hydrogen containing at least one carbon-carbon double bond
molecule consisting of carbon and hydrogen containing at least one carbon-carbon triple bond
cyclic molecule consisting of carbon and hydrogen with delocalized alternating carbon-carbon single and double bonds, resulting in enhanced stability
branch or functional group that replaces hydrogen atoms in a larger hydrocarbon chain
occur when two reactants exchange parts to give two new products
reactions in which a double carbon-carbon bond forms a single carbon-carbon bond by the addition of a reactant. Typical reaction for an alkene.

