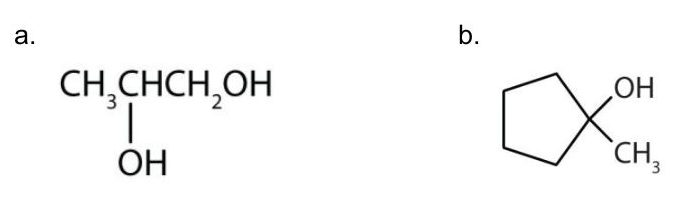23.1 Alcohols – Structure, Naming and Classification
Learning Objectives
By the end of this section, you will be able to:
- Identify the general structure for an alcohol.
- Identify the structural feature that classifies alcohols as primary, secondary, or tertiary.
- Name alcohols with both common names and IUPAC names
Incorporation of an oxygen atom into carbon- and hydrogen-containing molecules leads to new functional groups and new families of compounds. When the oxygen atom is attached by single bonds, the molecule is either an alcohol or ether.
Alcohols are derivatives of hydrocarbons in which an –OH group has replaced a hydrogen atom. Although all alcohols have one or more hydroxyl (–OH) functional groups, they do not behave like bases such as NaOH and KOH. NaOH and KOH are ionic compounds that contain OH– ions. Alcohols are covalent molecules; the –OH group in an alcohol molecule is attached to a carbon atom by a covalent bond.
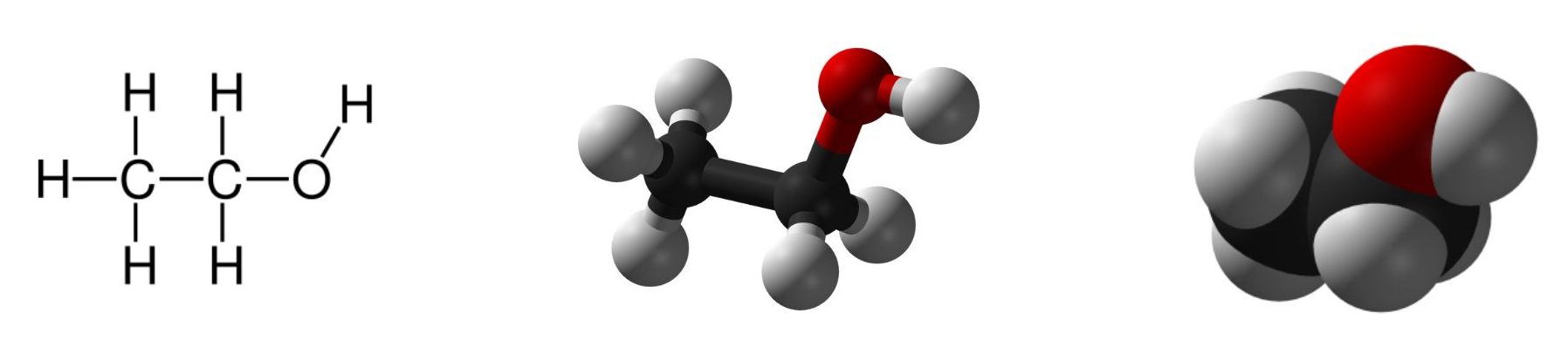
We often represent alcohols by the general formula ROH, where R is an alkyl group. Alcohols are common in nature. Most people are familiar with ethyl alcohol (ethanol) (Figure 23.1a.), the active ingredient in alcoholic beverages, but this compound is only one of a family of organic compounds known as alcohols. The family also includes such familiar substances as cholesterol and the carbohydrates. Methanol (CH3OH) and ethanol (CH3CH2OH) are the first two members of the homologous series of alcohols.
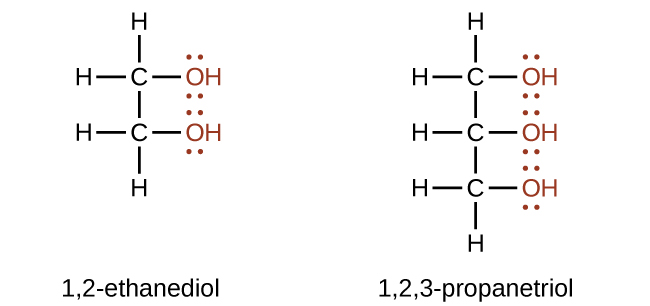
Alcohols with two -OH groups on adjacent carbon atoms are commonly known as glycols. Additional examples include 1,2-ethanediol (ethylene glycol, used in antifreeze, sweet colourless and somewhat viscous liquid) and 1,2,3-propanetriol (glycerin or glycerol, used as a solvent for cosmetics and medicines, sweet syrupy liquid) (Figure 23.1c.). Ethylene glycol is quite toxic. Because it is sweet, pets often lap up spills of leaked antifreeze from a garage floor or driveway. Sometimes people, especially children, drink it. The oxidation of ethylene glycol by the liver results in calcium oxalate crystals forming in the kidneys which causes renal damage and possibly death. This is not true of propylene glycol which is essentially nontoxic, and it can be used as a solvent for drugs and as a moisturizing agent for foods.
Nomenclature of Alcohols
According to the International Union of Pure and Applied Chemistry (IUPAC), alcohols are named by changing the ending of the parent alkane name to –ol. Here are some basic IUPAC rules for naming alcohols:
- The longest continuous chain (LCC) of carbon atoms containing the OH group is taken as the parent compound—an alkane with the same number of carbon atoms. The chain is numbered from the end nearest the OH group.
- The number that indicates the position of the OH group is prefixed to the name of the parent hydrocarbon, and the –e ending of the parent alkane is replaced by the suffix –ol.
- In 2013, IUPAC adopted new nomenclature guidelines that require the position number to be placed as an “infix” rather than a prefix. For example, the new name for 2-propanol would be propan-2-ol. Widespread adoption of this new nomenclature will take some time.
- In cyclic alcohols, the carbon atom bearing the OH group is designated C1, but the 1 is not used in the name.) Substituents are named and numbered as in alkanes.
- If more than one OH group appears in the same molecule (polyhydroxy alcohols), suffixes such as –diol and –triol are used. In these cases, the –e ending of the parent alkane is retained.
Example 23.1a
Give the IUPAC name for each compound.
-
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0). -
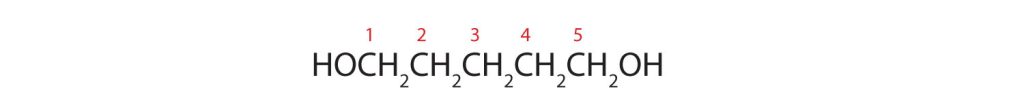
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
Solutions:
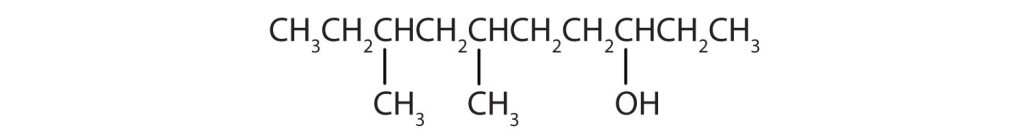
- Ten carbon atoms in the LCC makes the compound a derivative of decane (rule 1), and the OH on the third carbon atom makes it a 3-decanol (rule 2).
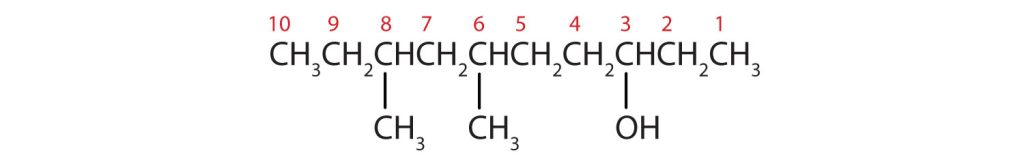
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0). The carbon atoms are numbered from the end closest to the OH group. That fixes the two methyl (CH3) groups at the sixth and eighth positions. The name is 6,8-dimethyl-3-decanol (not 3,5-dimethyl-8-decanol).
- Five carbon atoms in the LCC make the compound a derivative of pentane. Two OH groups on the first and fifth carbon atoms make the compound a diol and give the name 1,5-pentanediol (rule 3).
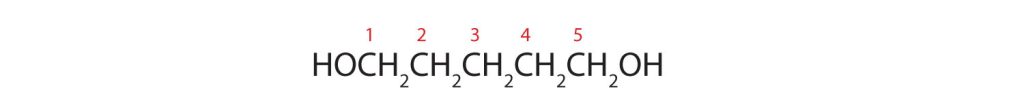
(credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0).
Exercise 23.1a
Name the following molecule:
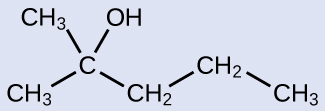
Check Your Answer:[1]
Exercise source: General Chemistry 1 & 2, CC BY 4.0.
Exercise 23.1b
Exercise source: Introduction to Chemistry: GOB(V. 1.0)., CC BY-NC-SA 3.0.
Draw the structure for each compound.
- hexan-2-ol
- 3-methyl-2-pentanol
Solution:
a) The ending –ol indicates an alcohol (the OH functional group), and the hex– stem tells us that there are six carbon atoms in the LCC. We start by drawing a chain of six carbon atoms: –C–C–C–C–C–C–.
The 2 indicates that the OH group is attached to the second carbon atom.
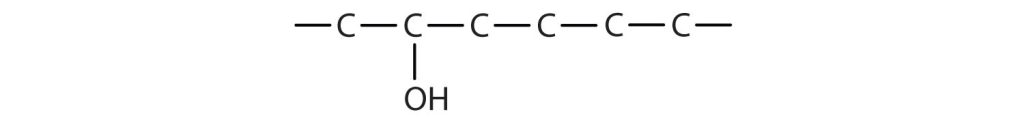
Finally, we add enough hydrogen atoms to give each carbon atom four bonds.
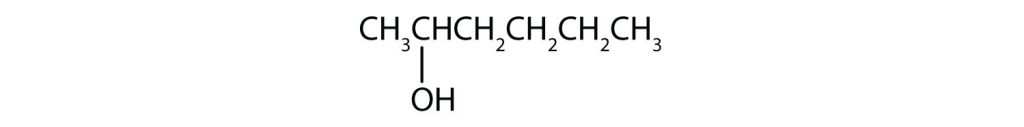
b) Pent- means 5 carbon chain. The numbers indicate that there is a methyl (CH3) group on the third carbon atom and an OH group on the second carbon atom.
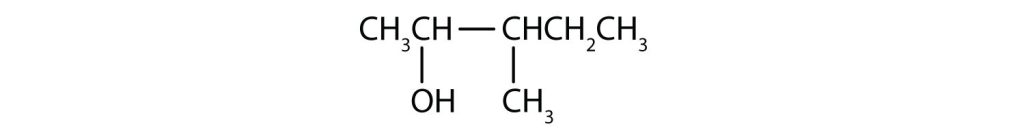
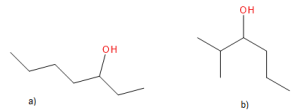
Exercise 23.1c
Draw the structure for each compound.
- heptan-3-ol
- 2-methyl-3-hexanol
Check Your Answers:[3]
Source: Exercise 23.1c is adapted from Basics of GOB Chemistry (Ball et al.) , CC BY-NC-SA 4.0, with images drawn by Samantha Sullivan Sauer using Biovia Draw, CC BY-NC 4.0.
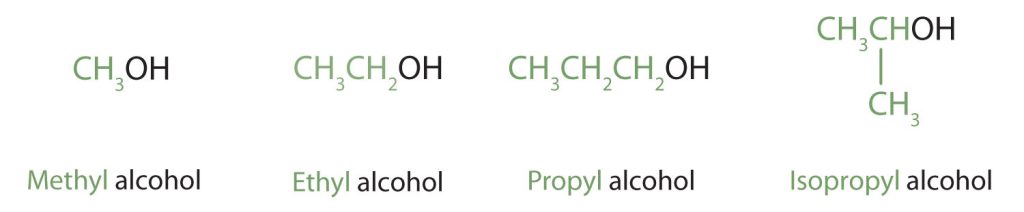
Alcohols with one to four carbon atoms are frequently called by common names, in which the name of the alkyl group is followed by the word alcohol (Figure 23.1d.). Structural formula of methyl alcohol, ethyl alcohol, propyl alcohol, and isopropyl alcohol with the methyl, ethyl propyl, and isopropyl groups are highlighted in green in Figure 23.1c.
Classification of Alcohols
Some of the properties of alcohols depend on the number of carbon atoms attached to the specific carbon atom that is attached to the OH group. Alcohols can be grouped into three classes on this basis.
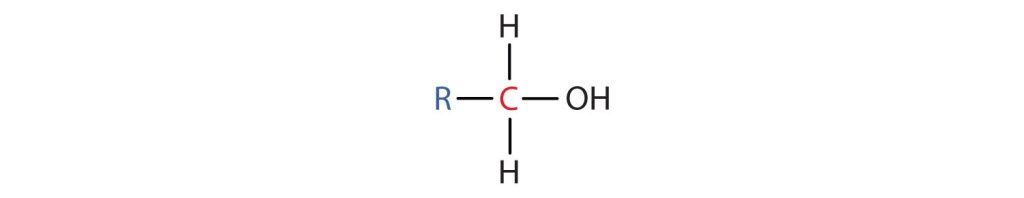
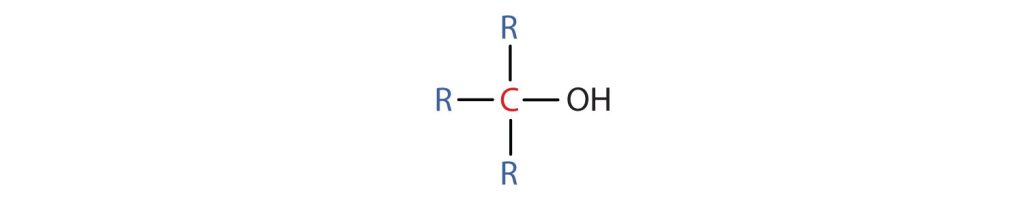
- A primary (1°) alcohol is one in which the carbon atom (in red) with the OH group is attached to one other carbon atom (in blue) (Figure 23.1e.). Its general formula is RCH2OH.
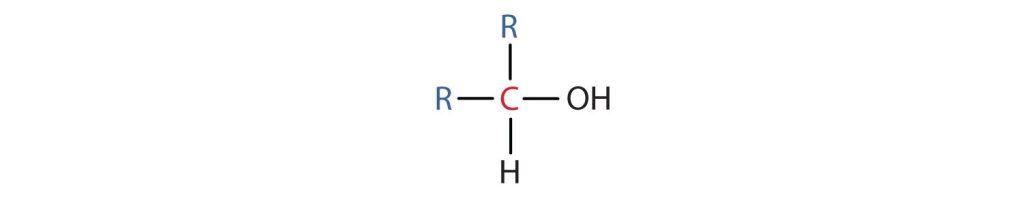
- A secondary (2°) alcohol is one in which the carbon atom (in red) with the OH group is attached to two other carbon atoms (in blue) (Figure 23.1f.). Its general formula is R2CHOH.
- A tertiary (3°) alcohol is one in which the carbon atom (in red) with the OH group is attached to three other carbon atoms (in blue) (Figure 23.1g.). Its general formula is R3COH.
Some of the common names reflect a compound’s classification as secondary (sec-) or tertiary (tert-). These designations are not used in the IUPAC nomenclature system for alcohols. There are four butyl alcohols corresponding to the four butyl groups: the butyl group (CH3CH2CH2CH2-), and three others in Figure 23.1h.
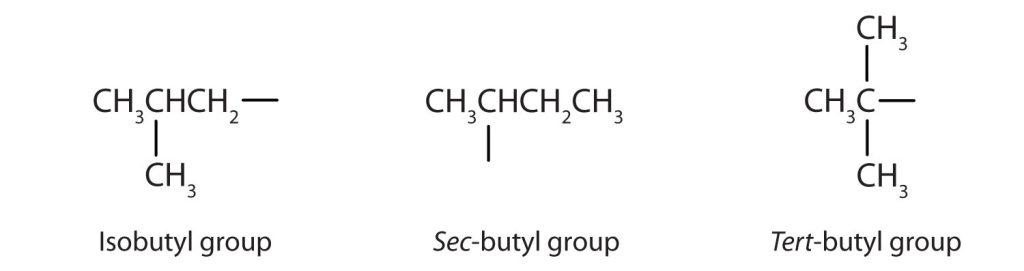
Table 23.1a. names and classifies some of the simpler alcohols.
| Condensed Structural Formula | Class of Alcohol | Common Name | IUPAC Name |
|---|---|---|---|
| CH3OH | — | methyl alcohol | methanol |
| CH3CH2OH | primary | ethyl alcohol | ethanol |
| CH3CH2CH2OH | primary | propyl alcohol | 1-propanol |
| (CH3)2CHOH | secondary | isopropyl alcohol | 2-propanol |
| CH3CH2CH2CH2OH | primary | butyl alcohol | 1-butanol |
| CH3CH2CHOHCH3 | secondary | sec-butyl alcohol | 2-butanol |
| (CH3)2CHCH2OH | primary | isobutyl alcohol | 2-methyl-1-propanol |
| (CH3)3COH | tertiary | tert-butyl alcohol | 2-methyl-2-propanol |
 |
secondary | cyclohexyl alcohol | cyclohexanol |
Indigenous Perspectives: Traditional Plant-Based Remedies
Many modern medicines have their roots in Indigenous traditional plant-based remedies. One such example is salicin. Salicin is a pain killing compound derived from the willow tree. Its structure has multiple -OH groups (Figure 23.1i.). On Turtle Island, Inuit harvest dwarf willow (a tiny bush that grows in the Arctic environment and a relative of the willow tree) as a source of pain relief (Figure 23.1i.). We now know that salicin is the active ingredient which is transformed in the human body to salicylic acid. Salicylic acid is related to acetylsalicylic acid commercially known under the brand name of Aspirin (Anderson & Rayner-Canham, 2022).

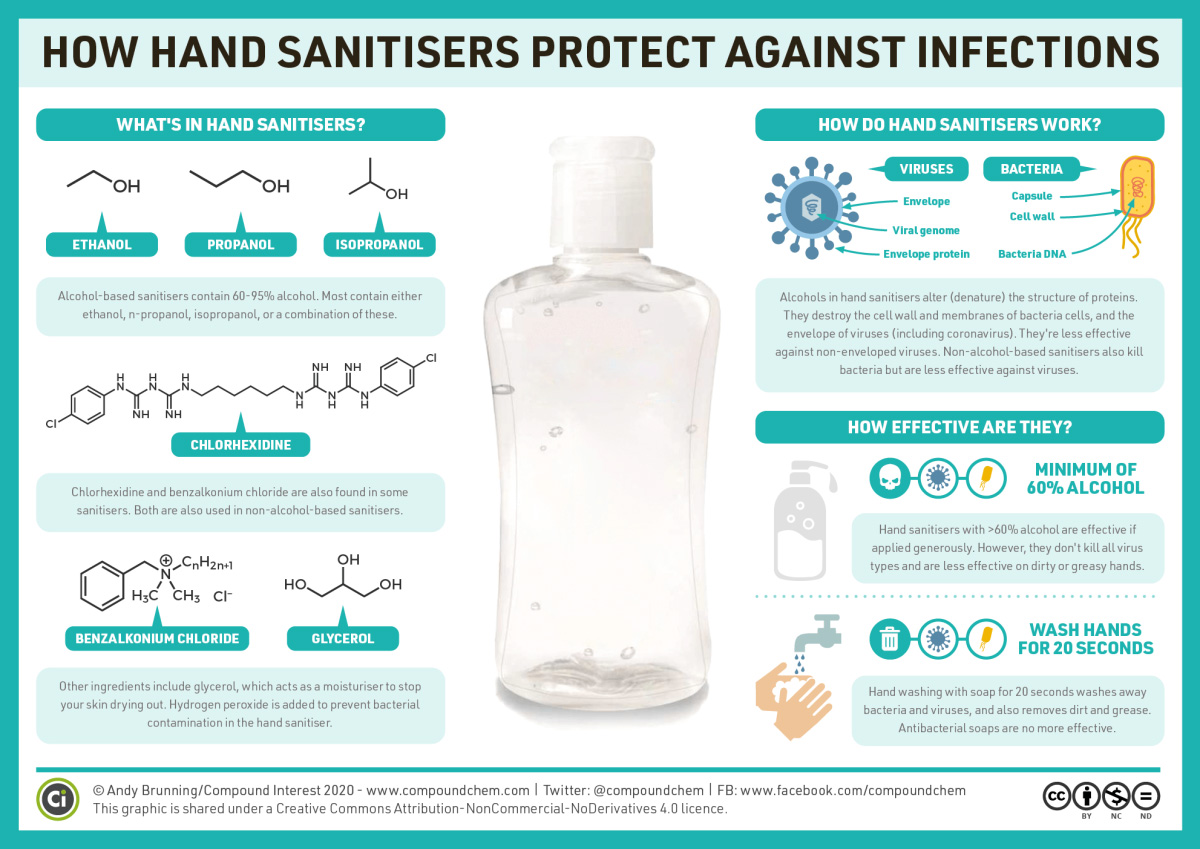
Spotlight on Everyday Chemistry: Hand Sanitizers
Hand sanitizers use alcohol to protect against infections including from viruses and bacteria. Infographic 23.1a. shows some of the alcohols used and how the alcohol helps prevent infection.
Attribution & References
Except where otherwise noted, this page is written and adapted by David Wegman and Samantha Sullivan Sauer from:
- “14.2 Alcohols – Nomenclature and Classification” In Basics of General, Organic, and Biological Chemistry (Ball et al.)by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, CC BY-NC-SA 4.0./ A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
- “18.2 Alcohols and Ethers” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “14.6: Glycols and Glycerol” In Basics of General, Organic, and Biological Chemistry (Ball et al.) by David W. Ball, John W. Hill, and Rhonda J. Scott via LibreTexts, licensed under CC BY-NC-SA 4.0. / A derivative of Introduction to Chemistry: GOB (v. 1.0), CC BY-NC 3.0.
References cited in-text
Anderson, C. C., & Rayner-Canham, G. (2022, Fall). Chemistry of the cure: Case studies of some Inuit remedies. Chem 13 News Magazine.
organic compound with a hydroxyl group (–OH) bonded to a carbon atom
alcohol in which the carbon atom with the OH group is attached to one other carbon atom
alcohol in which the carbon atom with the OH group is attached to two other carbon atoms
alcohol in which the carbon atom with the OH group is attached to three other carbon atoms