23.6 Ethers – Structure and Naming
Learning Objectives
By the end of this section, you will be able to:
- Describe the structural difference between an alcohol and an ether that affects physical characteristics of each.
- Name simple ethers.
- Describe the structure and uses of some ethers.
With the general formula ROR′, an ether may be considered a derivative of water in which both hydrogen atoms are replaced by alkyl or aryl groups (aryl means aromatic). It may also be considered a derivative of an alcohol (ROH) in which the hydrogen atom of the OH group is replaced by a second alkyl or aryl group (Figure 23.6a.).
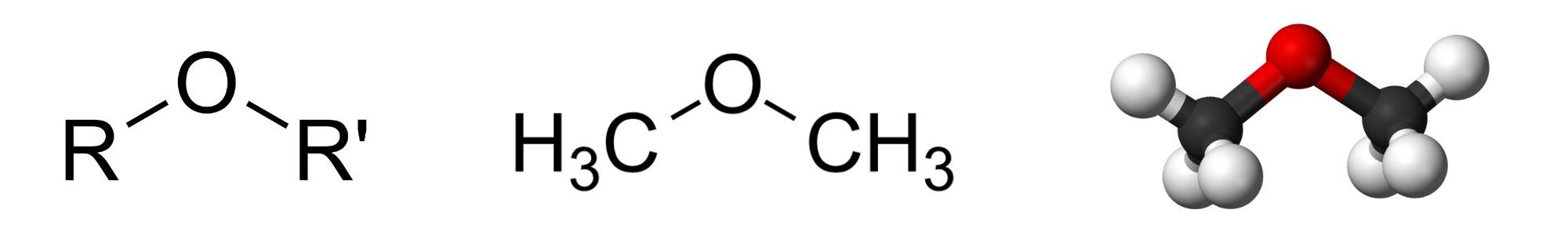
Naming Ethers
The IUPAC naming process of naming ethers involves separately naming each of the two groups attached to the oxygen atom.
- The group that has the longest continuous carbon chain is considered the parent chain and is named accordingly. (For example, if the longest chain consists of 3 carbons, the parent chain would be propane).
- The group attached to the oxygen that has the shorter chain is named as an alkoxy group, which replaces the “ane” ending with “oxy”. For example, a one carbon chain would go from methane to methoxy.
- Remaining substituents are numbered and named as in previous sections.
What is the IUPAC name for each ether?
- CH3CH2OCH2CH2CH3
- CH3CH2CH2CH2CH2CH2OCH2CH2CH2CH3
Solution:
- There is a two carbon group and a three carbon group on either side of the oxygen. The parent chain is based on the longer group (3 carbons = propane) and the alkoxy group is the shorter chain (2 carbons = ethoxy). The compound’s name would be ethoxypropane.
- There is a six carbon group and a four carbon group on either side of the oxygen. The parent chain is based on the longer group (6 carbons = hexane) and the alkoxy group is the shorter chain (4 carbons = butoxy). The compound’s name would be butoxyhexane.
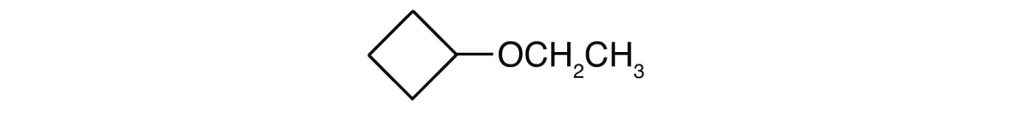
What is the IUPAC name for each ether?
- CH3CH2CH2CH2OCH2CH2CH2CH3
-
(Credit: Intro Chem: GOB (V. 1.0)., CC BY-NC-SA 3.0.)
Check Your Answers:[1]
Simple ethers also have common names, formed from the names of the groups attached to oxygen atom, followed by the generic name ether. If both groups are the same, the group name should be preceded by the prefix di-.
Example 23.6b
What is the common name for each ether?
- CH3–O–CH2CH2CH3
- CH3–O–CH3
- CH3CH2–O–CH2CH3
Solutions:
- methyl propyl ether
- dimethyl ether
- diethyl ether
Exercise 23.6b
What is the common name of this ether?
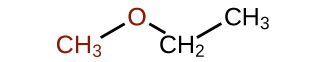
Check Your Answer:[2]
Source: Exercise 23.6b is adapted from General Chemistry 1 & 2, CC BY 4.0.
Physical Properties
Ether molecules have no hydrogen atom on the oxygen atom (that is, no OH group). Therefore, there is no intermolecular hydrogen bonding between ether molecules. As a result, ethers have quite low boiling points for a given molar mass. In fact, ethers have boiling points about the same as those of alkanes of comparable molar mass and much lower than those of the corresponding alcohols (Table 23.6a.).
| Condensed Structural Formula | IUPAC Name | Common Name | Molar Mass | Boiling Point (°C) | Intermolecular Hydrogen Bonding in Pure Liquid? |
|---|---|---|---|---|---|
| CH3CH2CH3 | propane | propane | 44 | –42 | no |
| CH3OCH3 | methoxymethane | dimethyl ether | 46 | –25 | no |
| CH3CH2OH | ethanol | ethyl alcohol | 46 | 78 | yes |
| CH3CH2CH2CH2CH3 | pentane | pentane | 72 | 36 | no |
| CH3CH2OCH2CH3 | ethoxyethane | diethyl ether | 74 | 35 | no |
| CH3CH2CH2CH2OH | butan-1-ol | butyl alcohol | 74 | 117 | yes |
Table source: “14.8: Ethers” In Basics of GOB (Ball et al.), CC BY-NC-SA 4.0.
Ether molecules do have an oxygen atom, however, and engage in hydrogen bonding with water molecules. Consequently, an ether has about the same solubility in water as the alcohol that is isomeric with it. For example, dimethyl ether and ethanol (both having the molecular formula C2H6O) are completely soluble in water, whereas diethyl ether and 1-butanol (both C4H10O) are barely soluble in water (8 g/100 mL of water).
Spotlight on Everyday Chemistry: General Anesthetics
A general anesthetic acts on the brain to produce unconsciousness and a general insensitivity to feeling or pain. Diethyl ether or ethoxyethane (CH3CH2OCH2CH3) was the first general anesthetic to be used. Diethyl ether is a colourless, volatile liquid that is highly flammable.
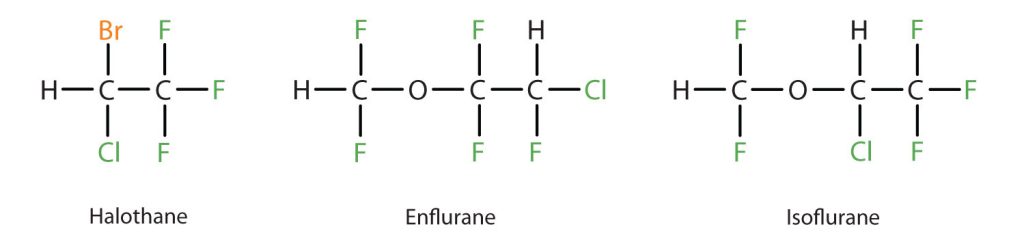
Diethyl ether is relatively safe because there is a fairly wide gap between the dose that produces an effective level of anesthesia and the lethal dose. However, because it is highly flammable and has the added disadvantage of causing nausea, it has been replaced by newer inhalant anesthetics, including the fluorine-containing compounds halothane, enflurane, and isoflurane (Figure 23.6c.). Unfortunately, the safety of these compounds for operating room personnel has been questioned. For example, female operating room workers exposed to halothane suffer a higher rate of miscarriages than women in the general population.
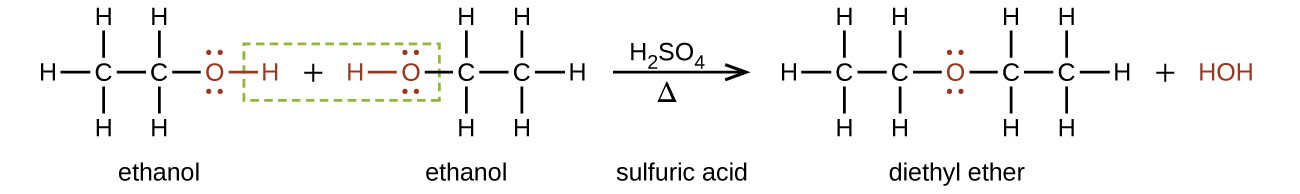
Ethers are produced from alcohols as previously described in this chapter. Figure 23.6d. shows an example of ether production.
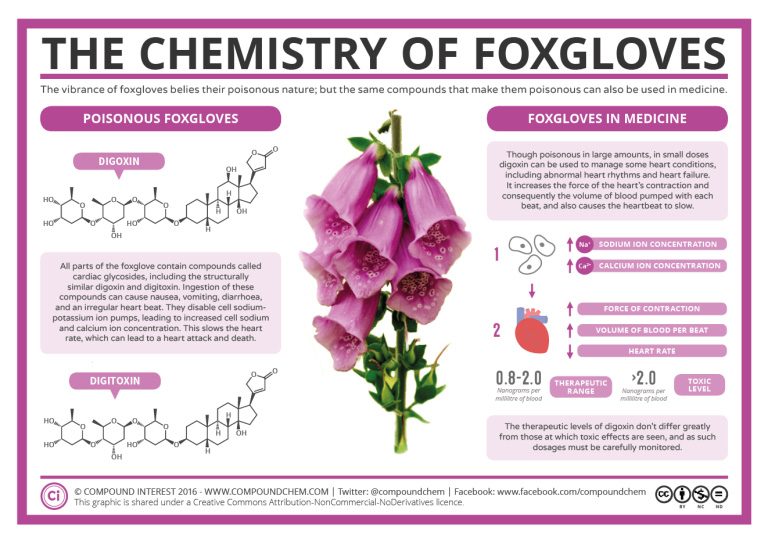
Spotlight on Everyday Chemistry: Foxgloves
Foxgloves are poisonous. The large biological molecules contained in the flowers have many ether functional groups. Read more about the chemistry of foxgloves in Infographic 23.6a.
Link to Enhanced Learning
For more support with naming ethers and their properties, see Ether naming and introduction (video) | Khan Academy.
Attribution & References
organic compound with an oxygen atom that is bonded to two carbon atoms

